TOURETTE
SYNDROME
 INTRODUCTION — Tourette syndrome (TS) is a
neurological disorder manifested by motor and phonic
tics with onset during childhood. Although TS is the
most common cause of tics, there are many potential
etiologies in the differential diagnosis, including
neuroacanthocytosis, certain drugs such as dopamine
receptor blocking drugs and cocaine, pervasive
developmental disorder and others.
INTRODUCTION — Tourette syndrome (TS) is a
neurological disorder manifested by motor and phonic
tics with onset during childhood. Although TS is the
most common cause of tics, there are many potential
etiologies in the differential diagnosis, including
neuroacanthocytosis, certain drugs such as dopamine
receptor blocking drugs and cocaine, pervasive
developmental disorder and others.
 PATHOGENESIS — TS was thought to be inherited in
an autosomal dominant pattern, but the mode of
inheritance may be more complex. In most cases a
bilineal transmission (inheritance from both
parents) is clearly evident. Although the genetic
basis remains elusive, several loci have been
identified as candidate susceptibility regions. The
disorder likely results from a disturbance in the
striatal-thalamic-cortical (mesolimbic) spinal
system, which leads to disinhibition of the motor
and limbic system.
PATHOGENESIS — TS was thought to be inherited in
an autosomal dominant pattern, but the mode of
inheritance may be more complex. In most cases a
bilineal transmission (inheritance from both
parents) is clearly evident. Although the genetic
basis remains elusive, several loci have been
identified as candidate susceptibility regions. The
disorder likely results from a disturbance in the
striatal-thalamic-cortical (mesolimbic) spinal
system, which leads to disinhibition of the motor
and limbic system.
The discovery of a mutation in the Slit and Trk-like
1 (SLITRK1) gene on chromosome 13q31.1 was a major
advance in the search for the elusive TS gene or
genes. The SLITRK1 gene is expressed in brain
regions previously implicated in TS (cortex,
hippocampus, thalamic, subthalamic and globus
pallidus nuclei, striatum, and cerebellum) and it
appears to play a role in dendritic growth. However,
it is not clear how the altered gene product leads
to the complex neurobehavioral disorder. This
mutation appears to be a rare cause of TS as it has
not been found in hundreds of TS patients tested.
Neuropathologic examinations have detected no
consistent brain abnormalities in patients with TS,
but a number of neuroimaging studies have found
evidence of structural changes in the brain. As an
example, a study using volumetric magnetic resonance
imaging (MRI) found that gray matter volumes in the
left frontal lobes were smaller in patients with TS
than in controls, supporting the loss of the normal
left > right asymmetry in this condition.
Although it has been proposed that antibodies to
basal ganglia neurons from Group A streptococcal
infection may contribute to pathogenesis of TS in
some patients, there is little or no evidence that
pediatric autoimmune neuropsychiatric disorder
associated with streptococcal infection (PANDAS)
plays a role in the development of TS. This issue is
discussed separately.
 CLINICAL FEATURES — Tics are the clinical
hallmark of TS. The onset typically is between two
and 15 years, although the diagnosis may be delayed
until 21 years in some cases. The disorder is
manifested by 11 years of age in 96 percent of
patients. In a large international registry of 3500
patients with TS, tics began at an average of 6.4
years. More males than females were affected
(4.3:1). TS was the sole diagnosis in only 12
percent of cases.
CLINICAL FEATURES — Tics are the clinical
hallmark of TS. The onset typically is between two
and 15 years, although the diagnosis may be delayed
until 21 years in some cases. The disorder is
manifested by 11 years of age in 96 percent of
patients. In a large international registry of 3500
patients with TS, tics began at an average of 6.4
years. More males than females were affected
(4.3:1). TS was the sole diagnosis in only 12
percent of cases.
Tics resolve by age 18 in about half of patients
with TS. Although tics may persist into adulthood,
their severity usually diminishes gradually over
time. Nonetheless, the most common cause of
"adult-onset" tics is TS that remits after puberty
and re-emerges as tics later in life. Other causes
of tics seen in adults are less common.
 Tics — Tics are sudden, brief, intermittent
movements (motor tics) or utterances (vocal or
phonic tics). Tics have been considered involuntary,
but tics can temporarily be voluntarily suppressed.
The tics in TS can be categorized as either simple
or complex. Simple tics include blinking, facial
grimacing, shoulder shrugging, and head jerking.
Many patients have complex sequences of coordinated
movements, including bizarre gait, kicking, jumping,
body gyrations, scratching, and seductive or obscene
gestures. Certain characteristics of the tics,
including the waxing and waning nature, the
irresistible urge before and relief after a tic, the
temporary suppressibility, and occurrence during
sleep, may result in the mistaken diagnosis of a
psychogenic disorder. One of the most characteristic
features of tics is the presence of premonitory
feelings or sensations, which are relieved by the
execution of the tic.
Tics — Tics are sudden, brief, intermittent
movements (motor tics) or utterances (vocal or
phonic tics). Tics have been considered involuntary,
but tics can temporarily be voluntarily suppressed.
The tics in TS can be categorized as either simple
or complex. Simple tics include blinking, facial
grimacing, shoulder shrugging, and head jerking.
Many patients have complex sequences of coordinated
movements, including bizarre gait, kicking, jumping,
body gyrations, scratching, and seductive or obscene
gestures. Certain characteristics of the tics,
including the waxing and waning nature, the
irresistible urge before and relief after a tic, the
temporary suppressibility, and occurrence during
sleep, may result in the mistaken diagnosis of a
psychogenic disorder. One of the most characteristic
features of tics is the presence of premonitory
feelings or sensations, which are relieved by the
execution of the tic.
 Involuntary vocalizations, ranging from
simple noises to coprolalia (obscene words),
echolalia (repetition of words), and palilalia
(repetition of a phrase or word with increasing
rapidity), frequently occur. Coprolalia occurs in
approximately 40 percent of cases. Many patients
also experience copropraxia (obscene gestures),
echopraxia (mimicking of gestures), bizarre thoughts
and ideas, thought fixation, compulsive ruminations,
and perverse sexual fantasies. Approximately
one-half of our patients have sleep complaints,
including restlessness, insomnia, enuresis,
somnambulism, nightmares, and bruxism. Motor tics
were recorded during sleep by polysomnography in
approximately two-thirds.
Involuntary vocalizations, ranging from
simple noises to coprolalia (obscene words),
echolalia (repetition of words), and palilalia
(repetition of a phrase or word with increasing
rapidity), frequently occur. Coprolalia occurs in
approximately 40 percent of cases. Many patients
also experience copropraxia (obscene gestures),
echopraxia (mimicking of gestures), bizarre thoughts
and ideas, thought fixation, compulsive ruminations,
and perverse sexual fantasies. Approximately
one-half of our patients have sleep complaints,
including restlessness, insomnia, enuresis,
somnambulism, nightmares, and bruxism. Motor tics
were recorded during sleep by polysomnography in
approximately two-thirds.
 Comorbidity — Comorbidity in TS is frequent. In
a large international registry of 3500 patients with
TS, comorbid conditions included attention deficit
hyperactivity disorder (ADHD) (60 percent),
obsessive compulsive disorder (OCD, 27 percent),
obsessive compulsive behavior (32 percent), learning
disorder (23 percent), and conduct
disorder/oppositional defiant disorder (15 percent).
Patients with more comorbidities were more likely to
have behavioral problems such as sleep difficulties,
coprolalia, self-injurious behavior, and anger
control problems. Motor and vocal manifestations
were more frequent in boys, whereas girls were more
likely to have behavioral problems such as OCD.
Comorbidity — Comorbidity in TS is frequent. In
a large international registry of 3500 patients with
TS, comorbid conditions included attention deficit
hyperactivity disorder (ADHD) (60 percent),
obsessive compulsive disorder (OCD, 27 percent),
obsessive compulsive behavior (32 percent), learning
disorder (23 percent), and conduct
disorder/oppositional defiant disorder (15 percent).
Patients with more comorbidities were more likely to
have behavioral problems such as sleep difficulties,
coprolalia, self-injurious behavior, and anger
control problems. Motor and vocal manifestations
were more frequent in boys, whereas girls were more
likely to have behavioral problems such as OCD.
The association of behavior disorders with tics in a
community-based study was similar to clinic-based
reports. In a study of school children aged 9 to 17
years, OCD, ADHD, anxiety disorders, and mood
disorders were significantly more common in children
with than without tics and with than without TS.
 Examination — The neurologic examination in
patients with TS is often normal except for the
presence of tics. However, some patients have
increased rates of normal blinking, subtle
oculomotor disturbances related to saccadic eye
movements, or other evidence of mild impairment of
motor control.
Examination — The neurologic examination in
patients with TS is often normal except for the
presence of tics. However, some patients have
increased rates of normal blinking, subtle
oculomotor disturbances related to saccadic eye
movements, or other evidence of mild impairment of
motor control.
 Neuroimaging — Standard anatomical neuroimaging
studies such as routine head CT and brain MRI are
unremarkable in patients with Tourette's syndrome.
However, volumetric magnetic resonance imaging
studies have found evidence of structural changes in
the brain. In addition, accumulating evidence
suggests that caudate nucleus volume loss may be a
disease marker of TS. A study using high resolution
MRI found that the average volume of caudate nucleus
was reduced in patients with TS by five to eight
percent compared with healthy controls. Furthermore,
a prospective longitudinal study of 43 children with
TS found that childhood caudate volume on MRI was
inversely associated with the severity of both tics
and obsessive-compulsive symptoms in late
adolescence and early adulthood.
Neuroimaging — Standard anatomical neuroimaging
studies such as routine head CT and brain MRI are
unremarkable in patients with Tourette's syndrome.
However, volumetric magnetic resonance imaging
studies have found evidence of structural changes in
the brain. In addition, accumulating evidence
suggests that caudate nucleus volume loss may be a
disease marker of TS. A study using high resolution
MRI found that the average volume of caudate nucleus
was reduced in patients with TS by five to eight
percent compared with healthy controls. Furthermore,
a prospective longitudinal study of 43 children with
TS found that childhood caudate volume on MRI was
inversely associated with the severity of both tics
and obsessive-compulsive symptoms in late
adolescence and early adulthood.
 DIAGNOSIS — The diagnosis of TS is based on the
clinical features of the disease, particularly the
presence of multiple motor and vocal tics, with
onset before age 21. The presence of vocal tics such
as grunting is required for the diagnosis. The
diagnosis is often supported by the presence of
coexisting behavioral disorders including attention
deficit hyperactivity disorder (ADHD) and obsessive
compulsive disorder (OCD). A family history of
similar symptoms also supports the diagnosis of TS.
DIAGNOSIS — The diagnosis of TS is based on the
clinical features of the disease, particularly the
presence of multiple motor and vocal tics, with
onset before age 21. The presence of vocal tics such
as grunting is required for the diagnosis. The
diagnosis is often supported by the presence of
coexisting behavioral disorders including attention
deficit hyperactivity disorder (ADHD) and obsessive
compulsive disorder (OCD). A family history of
similar symptoms also supports the diagnosis of TS.
The main entity in the differential diagnosis is
that of transient tics of childhood, which occur in
approximately 25 percent of normal children. The
ability to temporarily suppress tics is a feature of
TS that helps to differentiate tics from other
hyperkinetic movement disorders such as chorea,
dystonia, athetosis, myoclonus, and paroxysmal
dyskinesias.
As noted above, the mistaken diagnosis of a
psychogenic disorder may occur because of certain
characteristics of the tics in patients with TS,
including the waxing and waning nature, the
irresistible urge before and relief after a tic,
exacerbation during periods of stress and reduction
during mental concentration, and the temporary
suppressibility.
 Diagnostic criteria — There is no
confirmatory laboratory test; the diagnosis is based
on a set of clinical diagnostic criteria. The
Tourette Syndrome Classification Study Group
criteria for a definite diagnosis of TS are as
follows: Both multiple motor tics and one or more
phonic tics must be present at some time during the
illness, although not necessarily concurrently. Tics
must occur many times a day, nearly every day, or
intermittently throughout a period of more than one
year. Anatomical location, number, frequency, type,
complexity, or severity of tics must change over
time. Onset of tics before the age of 21 years.
Involuntary movements and noises must not be
explained by another medical condition. Motor tics,
phonic tics, or both must be witnessed by a reliable
examiner at some point during the illness or be
recorded by videotape or cinematography.
Diagnostic criteria — There is no
confirmatory laboratory test; the diagnosis is based
on a set of clinical diagnostic criteria. The
Tourette Syndrome Classification Study Group
criteria for a definite diagnosis of TS are as
follows: Both multiple motor tics and one or more
phonic tics must be present at some time during the
illness, although not necessarily concurrently. Tics
must occur many times a day, nearly every day, or
intermittently throughout a period of more than one
year. Anatomical location, number, frequency, type,
complexity, or severity of tics must change over
time. Onset of tics before the age of 21 years.
Involuntary movements and noises must not be
explained by another medical condition. Motor tics,
phonic tics, or both must be witnessed by a reliable
examiner at some point during the illness or be
recorded by videotape or cinematography.
 MANAGEMENT — Education about TS is important for
the patient, family, teachers, employers, and all
who interact with the patient. This should be the
first step in management of TS. Information and
resources are available online from the Tourette
Syndrome Association at www.tsa-usa.org.
MANAGEMENT — Education about TS is important for
the patient, family, teachers, employers, and all
who interact with the patient. This should be the
first step in management of TS. Information and
resources are available online from the Tourette
Syndrome Association at www.tsa-usa.org.
 Pharmacotherapy is indicated when symptoms of
TS are interfering with social interactions, school
or job performance, or activities of daily living.
Specific treatment of TS is guided by the need to
treat the most troublesome symptoms.
Pharmacotherapy is indicated when symptoms of
TS are interfering with social interactions, school
or job performance, or activities of daily living.
Specific treatment of TS is guided by the need to
treat the most troublesome symptoms.
 Dopamine agonists/antagonists — We treat tics
with drugs that block dopamine receptors, such as
fluphenazine, pimozide, and
tetrabenazine, which depletes neuronal
dopamine. These drugs appear to have a similar
response rate, reducing the frequency and intensity
of tics by approximately 60 to 80 percent. In our
experience, these drugs are more effective and
better tolerated than haloperidol. Tetrabenazine,
which depletes dopamine by inhibiting vesicular
monoamine transporter type 2 (VMAT2), is
particularly useful because it is as effective as
the typical neuroleptics, but it does not cause
tardive dyskinesias.
Dopamine agonists/antagonists — We treat tics
with drugs that block dopamine receptors, such as
fluphenazine, pimozide, and
tetrabenazine, which depletes neuronal
dopamine. These drugs appear to have a similar
response rate, reducing the frequency and intensity
of tics by approximately 60 to 80 percent. In our
experience, these drugs are more effective and
better tolerated than haloperidol. Tetrabenazine,
which depletes dopamine by inhibiting vesicular
monoamine transporter type 2 (VMAT2), is
particularly useful because it is as effective as
the typical neuroleptics, but it does not cause
tardive dyskinesias.
The use of pergolide, a mixed D1/D2/D3 dopamine
receptor agonist, has been suggested to treat
chronic tic disorders and TS. In one trial, 57
children, ages 7 to 17 years, with severe tics (Yale
Global Tic Severity Scale >30) were randomly
assigned to pergolide (0.15 to 0.45 mg per day) or
placebo in a two to one ratio. Pergolide resulted in
significantly lower scores of tic severity and
attention deficit hyperactivity disorder symptoms
than placebo. The drug was well tolerated and no
serious adverse events were observed. Similar
results were found in an earlier smaller trial.
Despite the results of these studies, it is found
that pergolide to be a powerful anti-tic drug. In
addition, valvular heart disease has been reported
in up to 33 percent of adult patients taking
pergolide. Thus, pergolide should probably be used
only for children with severe TS that is refractory
to other therapies.
The selective nonergoline dopamine agonist
ropinirole (0.25 to 0.5 mg twice a day) was
beneficial for all features of TS in a small
open-label study involving 15 children. This result
requires further confirmation in a large controlled
clinical trial.
 Botulinum toxin injection — Focal motor and
vocal tics may be treated with injections of
botulinum toxin into the affected muscles. This
treatment was safe and effective for the reduction
of tic frequency in a single randomized controlled
clinical trial.
Botulinum toxin injection — Focal motor and
vocal tics may be treated with injections of
botulinum toxin into the affected muscles. This
treatment was safe and effective for the reduction
of tic frequency in a single randomized controlled
clinical trial.
 Alpha adrenergic agonists and SSRIs — The
alpha adrenergic agonists (ie,
clonidine and guanfacine) and the selective
serotonin uptake inhibitors (SSRIs) may be helpful
in patients with predominant behavioral symptoms,
particularly impulse control problems and rage
attacks. The SSRIs also are effective in treating
associated OCD.
Alpha adrenergic agonists and SSRIs — The
alpha adrenergic agonists (ie,
clonidine and guanfacine) and the selective
serotonin uptake inhibitors (SSRIs) may be helpful
in patients with predominant behavioral symptoms,
particularly impulse control problems and rage
attacks. The SSRIs also are effective in treating
associated OCD.
 Attention deficit disorder and tics —
Attention deficit disorder with or without
hyperactivity associated with TS usually is treated
with central nervous system stimulants such as
methylphenidate or dextroamphetamine.
Attention deficit disorder and tics —
Attention deficit disorder with or without
hyperactivity associated with TS usually is treated
with central nervous system stimulants such as
methylphenidate or dextroamphetamine.
While it has been recommended that CNS stimulants be
used with caution because they may precipitate or
exacerbate tics, a well-designed trial did not
support the notion that methylphenidate worsens
tics. In one study, 136 children with attention
deficit hyperactivity disorder (ADHD) and a chronic
tic disorder (>90 percent with TS) were randomly
assigned to clonidine alone, methylphenidate alone,
clonidine plus methylphenidate, or placebo. The
results included Significant improvement of ADHD in
all treatment groups compared to placebo Tic
severity lessened in all treatment groups compared
to placebo. A similar proportion of patients with
worsening tics with methylphenidate, clonidine, and
placebo (20, 26, 22 percent, respectively). The
combination of clonidine and methylphenidate was
most effective in improving ADHD and lessening tic
severity. Drugs were well tolerated, although
clonidine was associated with moderate to severe
sedation in 28 percent of patients
 Transcranial magnetic stimulation — A
possible approach to improve symptoms is reduction
of hyperexcitability in the motor and premotor
cortex. In a small single-blinded,
placebo-controlled, crossover trial in patients with
TS, repetitive transcranial magnetic stimulation to
reduce activity in these areas
did not improve symptoms.
Transcranial magnetic stimulation — A
possible approach to improve symptoms is reduction
of hyperexcitability in the motor and premotor
cortex. In a small single-blinded,
placebo-controlled, crossover trial in patients with
TS, repetitive transcranial magnetic stimulation to
reduce activity in these areas
did not improve symptoms.
 Deep brain stimulation — Patients with
disabling tics that are refractory to optimal
medical management may be
candidates for deep brain stimulation of globus
pallidus, thalamus or other subcortical targets.
Deep brain stimulation — Patients with
disabling tics that are refractory to optimal
medical management may be
candidates for deep brain stimulation of globus
pallidus, thalamus or other subcortical targets.

Evidence to up to 2019 Apr 29
Sara C B Casagrande,1 Rubens G Cury,1 Eduardo J L
Alho,2 and Erich Talamoni Fonoff 2
Author information Copyright and License information
PMC Disclaimer
 Abstract
Abstract
Tourette’s syndrome (TS) is a neurodevelopmental
disorder that comprises vocal and motor tics
associated with a high frequency of psychiatric
comorbidities, which has an important impact on
quality of life. The onset is mainly in childhood
and the symptoms can either fade away or require
pharmacological therapies associated with
cognitive-behavior therapies. In rare cases,
patients experience severe and disabling symptoms
refractory to conventional treatments. In these
cases, deep brain stimulation (DBS) can be
considered as an interesting and effective option
for symptomatic control. DBS has been studied in
numerous trials as a therapy for movement disorders,
and currently positive data supports that DBS is
partially effective in reducing the motor and
non-motor symptoms of TS. The average response,
mostly from case series and prospective cohorts and
only a few controlled studies, is around 40%
improvement on tic severity scales. The ventromedial
thalamus has been the preferred target, but more
recently the globus pallidus internus has also
gained some notoriety. The mechanism by which DBS is
effective on tics and other symptoms in TS is not
yet understood. As refractory TS is not common, even
reference centers have difficulties in performing
large controlled trials. However, studies that
reproduce the current results in larger and
multicenter randomized controlled trials to improve
our knowledge so as to support the best target and
stimulation settings are still lacking. This article
will discuss the selection of the candidates, DBS
targets and mechanisms on TS, and clinical evidence
to date reviewing current literature about the use
of DBS in the treatment of TS.
Keywords: deep brain stimulation, DBS, Tourette’s
syndrome, tics
 Introduction
Introduction
Tourette’s syndrome (TS) is a neurobehavioral
disease characterized by motor and phonic tics often
associated with many behavioral comorbidities such
as obsessive– compulsive disorder (OCD),
attention-deficit hyperactivity syndrome, impulse
control, and autism spectrum disorders.1,2 According
to the last DSM-V criteria, TS is now classified as
a movement disorder under neurodevelopmental
disorders section and its diagnosis is based on the
persistent occurrence of at least one vocal and two
motor tics beginning before 18 years old and lasting
longer than 1 year excluding other causes.3 Tics are
defined as sudden, short, intermittent,
“semi-involuntary” movements and vocalizations (can
be suppressed temporarily) that are preceded by a
premonitory urge or impulse.1,4 The family history
of tics or behavior disorders is often positive.5 TS
patients also present with other psychiatric
comorbidities such as depression, anxiety and
impulsivity, sleep disorders, learning disorders,
and in some cases a self-injurious behavior.2
Typically, between 15 and 17 of age, the majority of
TS patients experience a decrease in frequency and
severity of tics. By early adulthood, about
three-quarters of children with TS will have
considerable improvement in symptoms and about 32%
will be tic-free. While it does not affect cognition
and the intellect itself, this condition can cause
significant functional and social burden, sometimes
affecting normal development in school and
professional activities. Treatment includes mostly
behavioral therapy and oral medications, alone or in
association. At present, there are a variety of
psychoactive medications that interact with dopamine
(typical but mostly atypical antipsychotic agents)
and non-dopamine systems (such as α2 agonists),
associated or not with behavioral therapy, and
psychoeducative interventions with responses ranging
from 30% to 85%.6 Botulinum toxin injections can be
effective in focal tics.7 However, there are
patients who do not benefit from medication either
due to poor response or due to unpleasant side
effects that further limit their use. This subset of
patients can evolve with the persistence of tics,2,8
thus becoming treatment-refractory and severely
disabled.9 In this scenario, deep brain stimulation
(DBS) can be considered as an additional therapeutic
option for symptom control since the clinical
benefits have been demonstrated and complications
are at low rate.9
DBS has become an established treatment for movement
disorders including Parkinson’s disease, essential
tremor, dys-tonia, and in some psychiatric
disorders.10 The first stereotactic surgical
treatment with thalamotomy on the
centromedian-parafascicular complex for TS was
introduced in 1970,11,12 and Vanderwalle et al
reported the first case of severe DBS in 1999.13
Since then, many case series have been
published,14–16 and also a few randomized clinical
trials have been conducted in this area.17–19 While
small and uncontrolled studies have demonstrated the
positive effects of stimulation on motor symptoms in
TS,20–24 its effects on Tourette psychiatric
comorbidities remain uncertain and the published
results are conflicting.25–27
The precise pathophysiology of TS is unknown, but
collective concepts include it among “brain
circuits” disorders. A closer look at system
dysfunctions suggests an overactivity in the basal
ganglia thalamo-cortical (BGTCC) loops that may
involve various networks, apparently involving a
wide range of parallel loops, from ventral and
mesolimbic structures to the sensory-motor
dorsolateral segments of the circuit.28–30 Overall,
DBS brain targets currently used for the treatment
of TS mostly resemble the targets that are earlier
used in focal ablative procedures such as the
thalamus, pallidum, and ventral striatum/ventral
capsule (VS/VC). Targets aim at the control of motor
and psychiatric symptoms.35
This article provides evidence on the applicability
of DBS in the treatment of therapy-refractory TS,
discussing the best candidates for surgery and
targets, and provides an overview of the mechanism
behind the modulation of neural circuits in TS.
Indication criteria for DBS in TS (who?)
The current clinical indication criteria for DBS in
a TS patient are based on clinical diagnosis, with
high tic severity scores and the presence of
symptoms, despite the use of at least three
different pharmacological drugs: 1) an
alpha-adrenergic agonist, 2) two dopamine
antagonists, and 3) a drug from at least one
additional class (eg, tetrabenazine or clonazepam).31
Although some recommendations use age as an
exclusion criterion (impeding DBS to subjects below
18 or 25), this should not be an absolutely strict
criterion (Table 1 and Figure 1).15,30 However, it
is recommended to consult a local ethics committee
when considering surgery for patients younger than
18 years of age.
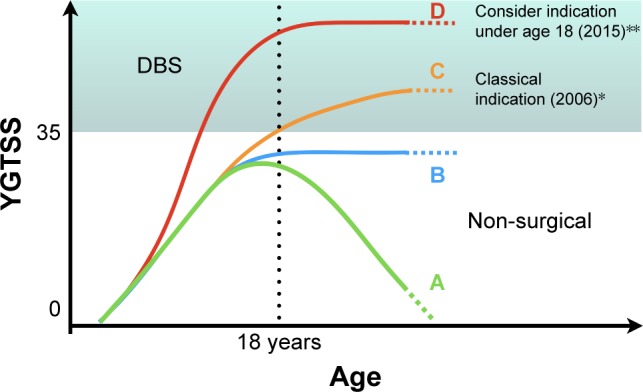
Figure 1
Diagram showing some of the possible clinical
evolution that can be interpreted as natural history
of TS or outcome from non-surgical therapeutic
interventions based on YGTSS as a severity measure.
This diagram intends to illustrate the current
indications for DBS according to earlier and latest
criteria. (A) Clinical resolution of TS symptoms.
(B) Presence of tics that do not resolve
spontaneously or are kept stable under non-surgical
treatments. (C) Classical indication for DBS based
on the severity of disease and age (18 years). (D)
Latest proposed indication for DBS based on severity
as a determinant factor even before 18 years of age.
Abbreviations: DBS, deep brain stimulation; TS,
Tourette’s syndrome; YGTSS, Yale Global Tic Severity
Scale.
Table 1
Clinical criteria for the indication of DBS in
Tourette’s syndrome
| |
2006 guidelines |
2015 MDS guidelines |
| Diagnosis |
DSM-IV
diagnosis of TS by expert clinician |
DSM-V diagnosis of TS by expert clinician |
| Age |
≥25 years old |
Age is not a strict criteria
* In patients ≤18 years old, a local ethics
committee should be involved |
| Tic severity
(measures) |
A. Severe tic disorder with
functional impairment
B. Scales: YGTSS >35/50
C. Document with standardized video
assessment |
A. Severe tic disorder with
functional impairment
B. Scales: YGTSS >35/50
C. Document with standardized video assessment A.
Severe tic disorder with functional impairment |
| Neuropsychiatric comorbidities |
A. Tics as the most
disable symptom
B. Stable and treated comorbid conditions
C. Scales: valid rating scales when
available |
A.
Tics as the most disable symptom
B. Stable and treated comorbid conditions
C. Scales: valid rating scales when available |
| Refractoriness
to conventional and optimal treatment |
A. Failed treatment trials from three
pharmacological classes:
A.1: alpha-adrenergic agonist
A.2: two dopamine antagonists (typical and atypical)
A.3: benzodiazepine
B. Evaluated for suitability of behavioral
interventions for tics |
A. Failed treatment trials
from three pharmacological classes:
A.1: alpha-adrenergic agonist
A.2: two dopamine antagonists (typical and atypical)
A.3: a drug from at least one additional class (eg,
clonazepam, tetrabenazine)
B. CBIT should be offered |
| Comorbid medical
disorders |
Stable for
6 months before DBS |
Stable for 6 months before DBS |
| Psychosocial
factors |
A. Adequate social support
without acute or subacute psychosocial stressors
B. Active involvement with psychological
interventions when necessary |
A. Adequate social
support without acute or subacute psychosocial
stressors
B. Active involvement with psychological
interventions when necessary
C. Caregiver available to accompany patient for
frequent follow-up
D. Psychogenic tics, embellishment, factitious
symptoms, personality disorders, and malingering
must be recognized and addressed |
| SI/HI |
Not
specifically addressed |
Documentation of
no active SI/HI for 6 months before surgery |
*Guideline changes in clinical indication criteria
for DBS in Tourette’s Syndrome (modified from
Schrock et al, 2015 15).
Abbreviations: DBS, deep brain stimulation; SI/HI,
suicidal/homicidal ideation; TS, Tourette’s
syndrome; YGTSS, Yale Global Tic Severity Scale;
CBIT, comprehensive behavioral intervention for
tics.
The exclusion criteria comprise major unstable and
non-treated psychiatric disorders, suicidal ideation
or psychiatric hospitalization preceding 6 months of
surgery, active dependence on alcohol or drugs, and
pregnancy and severe cognitive impairments.
Importantly, a multidisciplinary specialized DBS
team including a neurologist, psychiatrist,
neurosurgeon, neuropsychologist, speech therapist,
and physiotherapist should make all of these
assessments. Other exclusion factors include
significant structural lesion or abnormalities on
MRI.15 In addition, real expectations of motor
outcome and social support are essential when
referring patients for DBS.
Mechanisms of action of DBS in TS (how?)
The mechanism of action of DBS in movement disorders
has not yet been fully elucidated. There are many
theories that intended to explain how DBS interacts
with specific brain structures modulating
pathological oscillations on basal ganglia and
related circuits. DBS is mostly based on focalized
high-frequency stimulation (HF-DBS) in targets of
basal ganglia and thalamus involved in the mechanism
of movement disorders. The effect of HFS is
classically described as focal “lesion-like” effect
in most subcortical targets and stimulation of
fibers, including the ones used for the treatment of
TS. However, current concepts suggest that the
effect of DBS may be more complex. In 2016, Florence
et al published an article hypothesizing that the HF-DBS
induces ionic changes focalized in the region
surrounding the active tip, reversibly increasing
extracellular concentrations of potassium, which in
turn affects the dynamics of both cell bodies and
axons. This would contribute to the intermittent
excitation and inhibition of these elements,
reversibly interrupting local abnormal pathological
activity and consequently correcting circuit
irregularities.32
Regarding TS, when HF-DBS is applied in the
anteromedial globus pallidus internus (GPi), it
reduces the amplitude of tic-associated phasic
changes in the GPi. An animal study reported that
the suppression of the brain activity related to
tics was linked to a temporal locking of spiking
activity with the stimulation pulse, which induces
different patterns of inhibition and excitation in
affected cells.33 As previously mentioned,
dysfunction in the pathways related to the cortico-basal
ganglia integrative network has been associated with
vocal and motor tics; based on this, several
surgical targets have been proposed for the control
of motor and psychiatric symptoms.34 Unilateral
stimulation was found to be unsuccessful compared to
bilateral stimulation in a double-blind study.17
In TS, although acute effects of high frequency
stimulation (HFS) in deep structures are observed,
the major response after DBS, as observed in
idiopathic dystonia, is in general delayed and
gradually built-in.35 This suggests that the
mechanism of DBS in TS may be mediated by
neuroplastic changes in the circuit components.
Conversely, although a carry-over effect has been
observed, tics recur in most refractory cases after
DBS has been turned off, suggesting that the plastic
changes are of short or intermediate term. After the
DBS, as also observed in dystonia, the improvements
following TS DBS are delayed and are gradually
progressive.35
The role of dopaminergic modulation
Although different psychopharmacologic agents are
used to treat TS, the D2/3 receptor antagonists are
among the most effective. Therefore, this suggests
that the least the dopamine released in striatal
target neurons, the best the symptom control in TS.
In order to investigate this hypothesis, Vernalaken
et al reported an on/off stimulation experiment
using [18F] fallypride-positron emission tomography
scan during the steady phase of DBS treatment in a
TS patient showing a dramatic increase of endogenous
dopamine during off condition. So, bilateral
thalamic stimulation somehow induces a decrease in
dopamine in striatum.36 Corroborating this
hypothesis, a similar study involving three patients
also showed that DBS acts by modulating dopamine
transmission.37 It is possible that the stimulation
of the centromedian nucleus and substantia
periventricularis suppresses excitatory feedback
projections to motor and limbic circuits of the
striatum, thereby decreasing tics and consequently
improving behavioral disorders.13 Therefore, the
chronic circuit abnormalities present in TS are
probably related to the failure of cortical
inhibition to the basal ganglia “filter”, which in
turn will end-up in thalamic hyperactivity feeding
the pathological loop, originating the Tics.
The role of pathological oscillations
The analysis of activity dynamics recorded from
depth electrodes suggests that prominent oscillatory
brain activity at low frequencies (2–7 Hz) and in
alpha band (8–13 Hz), associated with decreased
thalamic beta activity, may be an important
component in the pathophysiology of TS.38–41
Comparisons of the effect of “on” and “off”
stimulation in the dynamics of these frequencies
suggested that HF-DBS is able to suppress the
abnormal oscillatory activity within the motor
cortico-basal ganglia network.38,39,41 Notable
increases in normalized gamma-band power activity
(25–45 Hz) were also observed, which indicate
clinical benefit. Correlation analysis showed that
the power of the gamma oscillations was inversely
associated with the degree of the TS symptoms, as
measured by the Yale Global Tic Severity Scale (YGTSS).42
All of these information are fundamental to the
development of advanced treatment strategies such as
closed-loop deep brain stimulation, also called
adaptive DBS (aDBS).43
The functional brain (cortical) modulatory effects
The pathophysiology of TS is still under
investigation, but some studies suggest overactivity
in the BGTCC.29,44 A functional study showed that TS
patients have a decrease in the fractional
anisotropy (FA) in many cortical areas, including
the pars opercularis of the left inferior frontal
gyrus, the medial frontal gyrus, and the right
cingulate gyrus. There was a positive correlation
between tic severity and FA scores in the corpus
callosum, thalamus, temporal gyrus, and
parahippocampal gyrus. Overall, the findings
advocated that tics are mostly produced by
alterations in prefrontal areas, thalamus, and
putamen.30
Regarding the effects of DBS in TS, few functional
studies have explored the white matter pathways and
the projections activated by stimulation in animal
models and patients, and, in general, they support
that good motor outcomes are related to the
activation of several fiber pathways and brain
cortical regions.39,45 The effect of DBS in TS, as
in other conditions, seems to be related to local
brain changes and also to the modulation of multiple
cortical distance areas (through structural and
functional connectivities).40
The closed-loop stimulation
Adaptive stimulation from closed-loop devices (aDBS)
depends on functional neural feedback through
variables recorded by DBS electrodes (such as
abnormal electro-graphic discharges or more recently
on neurochemical feedback).40,43,46 The term
“adaptative stimulation” was created with the
concept that some implantable generators are not
passive devices any more. Instead of only creating
and delivering monotonous trains of electrical
pulses, they perform recording and analysis of
neural signals and can be programmed to deliver,
stop, or change stimulation parameters when a
certain neural pattern takes place. Although the
studies that correlate recordings of deep brain
activity and simultaneous occurrence of symptoms are
still in the beginning stage, it seems that rather
than rapid activity related to every behavioral
event (tics), studies found changes in background
activity that correlates with periods of increased
tics, which helps to predict when those events will
arise. Therefore, detected changes in oscillatory
activity can lead to automatic responses from the
stimulator intended to suppress tic onset. When used
in a more dynamic way, the aDBS can adjust
stimulation parameters based on a feedback
information, leading to a more individualized
treatment.47,48
The main targets (where?)
A variety of brain targets have been proposed as
potential therapeutic targets for DBS in TS, along
the BGTCC circuit. In recent years, the centers of
DBS worldwide explored at least nine brain targets
for the treatment of TS:
centromedian-parafascicular-thalamic complex
(CM-Pf), the intersection point between centromedian
nucleus, periventricular substance, and inferior
ventro-oral nucleus in the thalamus (CM-Spv-Voi),
the posterior ventro-oral nucleus, the anterior
ventro-oral, and Voi, the GPi anteromedial or
posteroventral, the nucleus accumbens (NA), the
anterior internal capsule (ALIC), subthalamic
nucleus (STN), and globus pallidus externus (GPe)
(Figure 2 and Tables 2 and and 3).35,49–51 If TS is
considered a complex disease between movement and
psychiatric disorders (with anxiety and compulsive
symptoms), then both sensory-motor and
associative/limbic areas may be used as targets.
Therefore, of these options, regions of the medial
thalamus and the GPi are the most frequently used
targets probably because of historical reasons and
their involvement in motor and limbic pathways.
However, because of the close interconnection of
basal ganglia structures, the effects of stimulation
would block pathological signals in their local
network as well as reduce aberrant signals in other
connected structures associated with the mechanism
of TS.52–55 Other authors have suggested that
combining targets can provide additional benefits.56
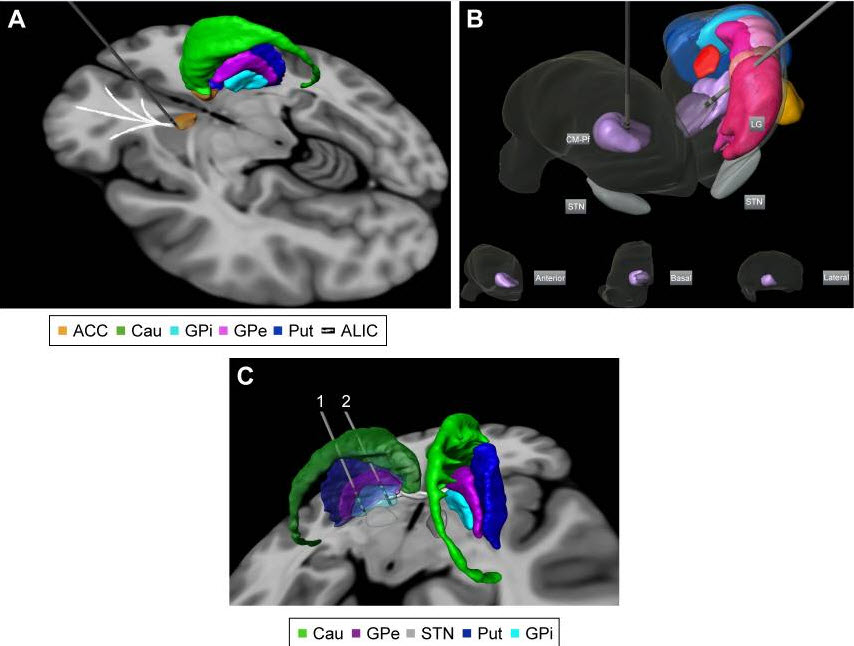
Figure 2
Targets proposed for DBS treatment in Tourette’s
syndrome: (A) anterior limb of internal capsule/accumbens;
(B) bilateral centromedian-parafascicular complex
targeted in an anterolateral thalamic view (basal,
anterior, and lateral thalamic views of the right
thalamus are displayed for localization within the
thalamus); and (C) different parts of GPi. Electrode
1 is located in the posteroventrolateral GPi and
electrode 2 is located in the anteromedial GPi. The
3D representations are histological postmortem
reconstructions of the nuclei from the University of
São Paulo – Würzburg Atlas of the Human Brain (Alho
et al, 201896).
Abbreviations: ACC, accumbens; Cau, caudate nucleus;
GPi, globus pallidus internus; GPe, globus pallidus
externus; Put, putamen; ALIC, anterior limb of
internal capsule; CM-Pf,
centromedian-parafascicular-thalamic complex; LG,
lateral thalamic group; STN, subthalamic group.
Table 2
Summary of the studies: level III evidence
Study Patients (n) Follow-up (months) Target
Outcomes
Maciunas et al, 200717 5 3
Centromedian-parafascicular and ventralis oralis
complex of the thalamus Three of five patients
showed improvement: mean pre-op YGTSS – 37.2, 3-mo
score – 28.2
Welter et al, 200877 3 20–60 Thalamus CM-Pf and GPi
30%–64% total YGTSS, 37%–41% tic severity subscale
with CM-Pf; 65%–96%, 67%–90% with GPi; 43%–76%,
16%–70% with combined; recurrence of tics during
sham but 32% improvement in 1 patient
Crossover study of CM-Pf vs GPi vs combined vs
sham(2 months per stimulation condition)
Porta et al, 200961 15 24
Centromedian-parafascicular and ventralis oralis
complex of the thalamus 5% improvement in tic
scores. No deleterious effect on cognition,
improvement in behavioral ratings
Kaido et al, 201197 3 12 Thalamus YGTSS decreased
from 42.7±2.7 (before DBS) to 26.0±1.7 (1 year after
DBS)
Ackermans et al, 201118 6 3, 6, 12 Thalamus
Improvement (37%) on the YGTSS scale (mean 41.1±5.4
vs 25.6±12.8, P=0.046)
After 1 year: significant improvement (49%) on the
YGTSS scale (mean 42.2±3.1 vs 21.5±11.1, P=0.028)
when compared with preoperative assessments
Okun et al, 2013116 5 6 Centromedian-parafascicular
and ventralis oralis complex of the thalamus YGTSS
decreased by 17.8 points (P=0.01), MRVRS decreased
by 5.8 points (P=0.01)
Motlagh et al, 201372 8 6–107 Thalamus (5) and GPi
(3)
Two in the sensorimotor portion and one in limbic
portion YGTSS decreased by 0–72%
Dong et al, 201270 1 22 with DBS
26 without DBS GPi 66.7% improvement
Schoenberg et al, 201525 5 5 Thalamus 24%
Huys et al, 201663 8 12 Ventral anterior and
ventrolateral motor parts of the thalamus YGTSS
motor, impairment, and total scores decreased by 51,
60, and 58%, respectively, compared to baseline
MRVRS score decreased by 58%
Significant improvement in quality of life and
global functioning measures were noted
Kefalopoulou et al, 201519 15 6 months blinded and
then 36 months unblinded GPi (anteromedial location)
15.3%–40.1%
YGTSS decreased by 12.4 between on and off states in
the blinded phase (P=0.048), YGTSS decreased by
23.8–48.9 points (P<0.0001) between baseline and
open-label phase
Servello et al, 201698 48 24 Thalamus – 42
aGPi – 2
NA – 4 78% of cases with >50% of improvement
Rossi et al, 201656 5 24 Thalamus CM-Pf 40%
Welter et al, 201776 16 3 aGPi No significant
difference in YGTSS score
Open in a separate window
Note: Summary of the main published studies on DBS
for the treatment of tics and Tourette’s syndrome.
Abbreviations: YGTSS, Yale Global Tic Severity
Scale; CM-Pf, centromedian-parafascicular-thalamic
complex; GPi, globus pallidus internus; aGPi,
anteromedial GPi; DBS, deep brain stimulation; NA,
nucleus accumbens; MRTRSS, Modified Rush Tic Rating
Scale Score total score.
Table 3
Summary of studies: level IV evidence
Study Patients (n) Follow-up (months) Target Outcome
Vandewalle et al, 199913 1 12 Thalamus Total
symptomatic improvement
Van der Linden et al, 2002 1 6 Medial thalamus and
GPi 80% Improvement with thalamus at high
intensities, 95% with GPi at lower intensities at 1
wk; GPi connected to implantable pulse generator (IPG),
with similar results at 6 mo
Visser-Vandewalle et al, 200364 3 8–60 Thalamus
Improvement of motor and vocal severe tics
Houeto et al, 200599 1 3, 5, 7, 9, 10 CM-Pf and GPi
65% total improvement on YGTSS, 77% improvement on
RVBTS after CM-Pf; 65% total impr on YGTSS, 67% impr
on RVBTS after GPi. Return to the baseline with sham
Stim; 70% total impr on YGTSS, 76% improvement on
RVBTS after CM-Pf + GPi.
Flaherty et al, 200582 1 18 ALIC/NA Symptomatic
improvement
Diederich et al, 200565 1 14 pGPi 71.6% tic/min (on
videotape) at 7 mo, 84.6% at 14 mo; 66.0% tic
increase at 14 mo “off”; 47.0% total improvement on
YGTSS (44.2% tic severity subscale); improved
premonitory urges – recurrence at 7 mo “off”
Gallagher et al, 2006100 1 Non-disclosed GPi
Improvement
Ackermans et al, 200678 2 12 CM, Spv, Voi in patient
1 GPi in patient 2 85.0% (tics/min on videotape) in
patient 1, 92.9% in patient 2; minor tics remained
in both patients; acute increase and decrease of
tics during “off” and “on,” respectively
Vilela Filho et al, 200785 2 23 GPe Symptomatic
improvement
Shahed et al, 200727 1 6 GPi posteroventral 76.0%
motor, 68.0% phonic tics (84.4% total on YGTSS);
21.4% RVBTRS
Bajwa et al, 200759 1 1, 4, 6, 14, 20, 24 CM, Spv,
Voi 66.2% YGTSS tic subscale; 98.0% reduced tic
frequency via hand-held counter
Kuhn et al, 200781 1 30 ALIC/NA 41.1% total on YGTSS,
50% RVBTRS at 30 mo
Zabek et al, 200880 1 Baseline, at 6 and 28 Right NA
26.7% at 1 wk, 74.2% at 6 mo, 79.7% at 28 mo
(tics/15 min via videotape); 50% tics in “off”
Shields et al, 2008101 1 18 VS/VC, thalamus 45%
Khun et al, 2008102 1 10 VS/VC 19.8% total on YGTSS
at 1 mo, 51.9% at 10 mo; coprolalia nearly resolved
Dehning et al, 200866 1 12 GPi 66.3% total on YGTSS
at 6 wk, 88.0% at 1 y (tics abolished)
Servello et al, 200860 18 3–18
Centromedian-parafascicular (CM-Pf) and ventralis
oralis complex of the thalamus YGTSS decreased from
33–48 to 7–22
Neuner et al, 200983 1 36 NA, ALIC 46.0% at 3 mo,
44.0% at 36 mo, 40% at 58 mo (total YGTSS), 60%,
58%, 57% (RVBTRS)
Servello et al, 200987 4 44/8–51 Internal capsule/NA
in patients with centromedian-parafascicular and
ventralis oralis complex of the thalamus (except one
patient with only internal capsule/NA leads) Two
patients showed at best mild improvement in OCD and
tic scores, two showed more clinically significant
improvement in OCD scores and functionality, with
limited effect on tics
Vernaleken et al, 200936 1 Non-disclosed GPi, CM-Pf,
DM No clinical improvement with GPi; 35.9% total on
YGTSS (22.2% motor and 40.0% vocal tics) with
CM-Pf/DM
Kuhn et al, 2009103 6 3–18 NA (n=2); GPi (n=2);
thalamus (n=1); caudate (n=1) 50% improvement n=3;
50% in N=2; non response in n=1 during
Dueck et al, 2009104 1 12 GPi Improvement in YGTSS
scores, but not substantial overall
Foltynie et al, 2009105 1 Non-disclosed GPi 88.7%
motor and 90% vocal tics/5 min at 3 and 6 mo;
reemergence of tics during “off” and of vocal tics
when trying to speak; inner tension remained
Martinez-Torres et al, 200979 1 12 STN 89% at 6 mo,
97% at 1 y (tics/10 min)
Ackermans et al, 201058 2 120/60 12
Centromedian-parafascicular and ventralis oralis
complex of the thalamus YGTSS decreased from a mean
of 42.3 prior to surgery to 21.5 on 1-y follow-up,
P=0.028
Marceglia et al, 2010106 7 24 CM-Pf, Vop 33%
Improvement in YGTSS (6 mo-2 y follow-up)
improvement in motor scale
Burdick et al, 201084 1 30 VS/VC No improvement in
tics, 120.0% (RVBTRS) and 115.2% (total YGTSS) at 6
mo
Lee et al, 2011107 1 18 Thalamus (CM-Pf) 81%
improvement in total tics count and 58% improvement
on YGTSS
Martínez-Fernández et al, 201175 5 3–24 GPi (two
patients with anteromedial location, two patients
with posterolateral location, one patient initially
with posterolateral switched after 18 mo to
anteromedial location) Mean YGTSS was 77.8 at
baseline and 54.2 at last follow-up, mean MRVRS was
28.3 at baseline and 15.7 at last follow-up,
Tourette Sindrome Quality of Life was 61.7 at
baseline and 28.5 at last follow-up
Dehning et al, 201171 4 5–48 GPi (posteroventrolateral
location) Two patients responded with >80% reduction
in tics, two patients did not respond
Kuhn et al, 2011108 2 12 Thalamus (CM-Pf) ↑100%/67%
Savica et al, 201223 3 12 Thalamus (CM-Pf) ↑70%
Dong et al, 201270 2 12 GPi D ↑58.5%/53.1%
Cannon et al, 201274 11 4–30 GPi (anteromedial
location) One patient did not respond; mean YGTSS
was 84.45 before surgery and 42.55 at 3 mo, mean
TSQOL was 39.09 before surgery and 79.09 at 3 mo
Maling et al, 201240 5 6 Centromedian-parafascicular
and ventralis oralis complex of the thalamus YGTSS
decreased by 1%–41%; noted correlation between
gamma-band activity change and YGTSS change after
DBS
Hwynn et al, 2012109 1 1, 3, 6, 9, 12, 24, 36 GPi
Improvement in tics and dystonia
Porta et al, 201262 18 60–72
Centromedian-parafascicular and ventralis oralis
complex of the thalamus Mean YGTSS was 80.83 prior
to surgery and 22.11 at the extended follow-up
(P<0.001) 72.6% Improvement.
Piedimonte et al, 201386 1 6 GPe ↑70.5%
Dehning et al, 201467 6 12–60 GPi (posteroventrolateral
location) Two patients were non-responders, mean
YGTSS was 90.2 prior to surgery and 29.5 at last
follow-up (P=0.001); TSQOL was 88.75 prior to
surgery and 7.75 at last follow-up (one person did
not fill TSQOL)
Huasen et al, 2014110 1 12 GPi anteromedial 55%
Zhang et al, 201424 13 13–80 GPi (posterolateral
location) Mean YGTSS decreased by 52.1% at last
follow-up, mean TSQOL improved by 45.7% at last
follow-up
Sachdev et al, 201422 17 48 GPi anteromedial 38%
Patel & Jimenez-Shahed, 2014111 1 14 GPi 47%
Zekaj et al, 2015112 1 72 Thalamus 58.2% improvement
during “off” condition
Testini et al, 2016113 12 Median 26 Thalamus (CM-Pf)
54% improvement
Smeets et al, 201668 5 1–12–38 Anterior internal
globus pallidus YGTSS was significantly lower than
the preoperative score (42.2±4.8 vs 12.8±3.8,
P=0.043). No significant difference in the secondary
outcomes was found; however, there was an
improvement at an individual level for
obsessive–compulsive behavior
Cury et al, 201626 1 18 Thalamus (CM-Pf) 70.5%
Zhang et al, 201424 24 12 GPi 56%
Dwarakanath et al, 2017114 1 Non-disclosed GPi
anteromedial 72%
Hauseux et al, 201788 3 52 GPi posteroventral + GPe
GPi posteroventral + GPi posteroventral + thalamus
(CM-Pf) + NA Symptoms improvement
Smeets et al, 2017115 7 12–78 Thalamus (CM-Pf)
Improvement from 9% to 88.1%
Open in a separate window
Note: Summary of the main published studies on DBS
for the treatment of tics and Tourette’s syndrome.
Abbreviations: mo, month; y, year; wk, week; YGTSS,
Yale Global Tic Severity Scale; CM-Pf,
centromedian-parafascicular-thalamic complex; GPi,
globus pallidus internus; DBS, deep brain
stimulation; STN, subthalamic nucleus; GPe, globus
pallidus externus; NA, nucleus accumbens; ALIC,
anterior internal capsule; VS/VC, ventral
striatum/ventral capsule; pGPi, posteroventral GPi;
OCD, obsessive–compulsive disorder.
Thalamus
Several studies and clinical trials of thalamic DBS
indicated that bilateral CM-Pf and Voi stimulation
provide a beneficial therapeutic role in TS for both
tic severity (motor via CM) and psychiatric symptoms
(limbic via Pf).14,57,58 This target was introduced
by the ablative surgery of Hassler and Dieckmann in
1970.11 Based on this, Vandewalle et al (1999)13
published the first report of thalamic DBS for a
42-year-old man with refractory TS. They applied
high-frequency continuous bilateral stimulation (4
V, 130 Hz, 450 µs). Preoperatively, he had 38 tics
per minute; at 4 months, after 12 hours in the
off-stimulation condition, only eight tics per
minute were counted; all tics subsided 5 minutes
after the stimulation was switched on except for
some excessive eye-blinking. After 1 year,
stimulation of 1.5 V was sufficient to abolish his
tics. In long term (5 years), the results of these
patients were published in 2003, together with two
more cases, and the results showed an average
improvement of 72. 2%–90% with no serious
complications. Obsessive–compulsive and
self-injurious behaviors completely disappeared in
all patients.21 Ten years after surgery, patient 1
showed sustained improvement in tic frequency with
no change in cognition.59
The first blinded trial on thalamic stimulation for
TS was conducted by Maciunas et al with five TS
patients in 2007. Three of the five presented with
50% reduction in tics severity after open-label
stimulation at 3 months. There was a marked
improvement according to all primary (modified Rush
Video-Based Rating Scale) and secondary outcome
measures (OCD, depression, and anxiety scales).17
Also, this study showed that unilateral stimulation
did not appear to be beneficial. Bajwa et al
reported a patient who showed improved total tic
score and Yale-Brown Obsessive-Compulsive Scale (YBOCS)
by a mean of 66%, evaluated 24 months after the
surgery.59
In a larger series of study conducted by Servello et
al, 15 of 18 patients showed YGTSS improvement
between 24% and 79%, with improvement in
comorbidities. Stimulation was performed with
current between 1 and 5 mA, 100 Hz, and a pulse
width of 60 µs.60 This same group of authors later
published their long follow-up results of 36 TS
patients with different DBS targets. Most of the
patients had thalamic DBS, and significant
improvements were documented. Servello et al also
published their results of a cohort of 48 TS DBS
patients. In 40 of them, the thalamus was the target
chosen. The target was different than that chosen by
Vandewalle et al (1999)117 because it is located 2
mm more anteriorly. The authors stated that this can
stimulate the limbic fibers and, consequently, act
on the behavioral components of TS. The patients had
a mean improvement of 47.5% in YGTSS after DBS and
kept at 35% improvement at the final followup. After
2 years of thalamic DBS, Porta et al reported a
clinical follow-up of 15 patients, whose YGTSS
scores decreased from 76.5 to 36.6. The
neuropsychiatric scales also improved.61 The same
group of authors published a longer follow-up study
(5–6 years) of the same cohort and showed a mean
YGTSS improvement of 73% and YBOCS of 42%. However,
compared with the results at 2 years, they
demonstrated some long-term difficulties.62
Similarly, in a 2-year follow-up study, Rossi et al
showed that a 30% improvement in the total YGTSS
scores (range 10%–58%) was observed in four CM-Pf
DBS cases across the cohort.56 In 2011, Ackermans et
al studied six TS patients in a double-blind
randomized trial in which chronic stimulation was
delivered bilaterally in the CM-Spv-Voi complex (1–6
mA, 130 Hz, 60 µs). The authors reported
improvements in the YGTSS scores during the on- vs
off-stimulation conditions. The YGTSS improved by
37% and remained after 1-year open-label follow-up,
with a 49% improvement reported.18 In 2012, Savica
et al described three patients with TS who underwent
CM-Pf DBS with an excellent clinical outcome (mean
reduction in the YGTSS of 70%) at 1-year
follow-up.23 Recently, two other prospective trials
presented five and eight intractable TS
patients.25,63 The first study indicated that
bilateral CM-Pf DBS provided treatment for medically
refractory TS with concomitant improvement in
depression and anxiety with no neuropsychological
morbidity.27 In the second study the patients were
treated with DBS of the Voa-Vop, indicating a
significant beneficial effect on psychiatric and
motor symptoms of TS. In addition, the presence of
compulsive behavior, anxiety, and emotional
deregulation before surgery appeared to be
significant predictors of good outcome after DBS.63
Globus pallidus internus
Posteroventral GPi (pGPi) The GPi stimulation
affects both motor and limbic pathways; however,
this specific target has been used for motor
symptoms especially for Parkinson’s disease and
dystonia. Accordingly, pGPi as a target for DBS has
been considered for the treatment of hyperkinetic
movements as well as in TS. There are a number of
case reports and trials using this target in TS. The
first pallidal stimulation in TS was reported by Van
der Linden et al.64 The patient underwent both pGPi
and thalamic DBS and showed 80% reduction in tics
with thalamic stimulation and 95% with pallidal
stimulation maintained for 6 months. In 2005,
Diederich et al reported progressive improvements in
tic frequency reaching 73% within 14-month follow-up
after pGPi together with improvement in depressive
and anxiety symptoms.65 Dehning et al reported 87%
improvement on YGTSS 1 year after bilateral pGPi
electrodes in four patients with refractory TS with
maintenance of the benefit for 4 years. The authors
observed that the patients who improved after DBS
had also shown prior response to electroconvulsive
therapy.66,67 More recently, pGPi stimulation for
the treatment of TS has been performed more
frequently with substantial motor tics.24,25,68 The
youngest TS patient ever treated by DBS received
leads in the pGPi (Shahed et al’s study), who showed
84% improvement on YGTSS after 6 months.27 That
patient was followed for 5 years and later reported,
with other two patients (followed for 4 and 2
years), to show good results. Over the longitudinal
evaluation, stimulation parameters were considered
high (mean values 4.9 V, 198 ms, 168 Hz) and
rechargeable batteries were eventually used.
Transient reduction and gradual retitration of
stimulation parameters were sometimes required after
the battery exchange. Overall, clinical improvement
was maintained over the treatment period. The
authors demonstrated that the benefits over symptom
could be maintained for up to 5 years.27 There are
also other series of cases reported in the
literature with positive results.69–72
Anteromedial GPi (aGPi) The GPi is functionally
divided into an anteromedial region that is part of
the associative/limbic part of the BGCTCC circuit.73
There are studies that report good outcomes in
stimulating the aGPi (the limbic subregion). This
involves the limbic loops in tic expression.74,75
More recently, Akbarian-Tefaghi described 15
patients with aGPi DBS for severe TS and explored
whether a specific anatomical location within the
aGPi correlated with motor outcome for tics,
obsessive-ompulsive behavior (OCB), and mood. They
demonstrated that the region within the ventral
limbic GPi – specifically on the medial medullary
lamina in the pallidum at the level of the anterior
comissure-posterior comissure line (AC-PC Line) –
was significantly associated with improved tics, but
not mood or OCB outcome.46 Another recent randomized
clinical trial by Welter et al involved 19 patients
and showed that aGPi DBS was insufficient to
decrease tic severity after 3 months. Future
research is warranted to explore the effectiveness
of aGPi DBS over longer follow-up and optimal
stimulation parameters as well as to study potential
predictors of the therapeutic response.76
Comparative studies
Gpi vs thalamus/Gpi and thalamus In the search for
an optimal surgical target, a few studies have
compared the outcomes of stimulation in the limbic
regions of the GPi and medial thalamus.77,78 A
randomized blinded study evaluated the efficacy of
stimulating the CM-Pf vs the ventromedial GPi in
patients with TS refractory to medical treatment.
Bilateral stimulation of the GPi reduced tic
severity by 65%, 96%, and 74% in patients 1, 2, and
3, respectively, whereas CM-Pf DBS reduced tic
severity by 64%, 30%, and 40%, respectively. The
association of thalamic and pallidal stimulation
showed no further reduction in tic severity. The
tics returned during the sham condition.77
aGpi vs pGpi/aGpi vs pGpi Martinez-Fernandez et al
studied five TS DBS patients – three of them
target-implanted in the pGPi and the other two in
the aGPi. All patients experienced improvements in
tic severity but to variable extents. The YGTSS
scores reduced by 29% (before = 77.8, after = 54.2)
and the YBOCS reduced by 34% (before = 16.3, after =
10.8) – this effect was sustained until the last
follow-up. The authors stated that the anteromedial
part of GPi appeared to be a more effective
target.75
Other targets The STN, GPe, ALIC, and NA also
referred as VS/VC can act as alternative targets for
TS stimulation, and a few reports have been reported
on this topic.
STN: A case report was published in 2009 of a
patient who had Parkinson’s disease (PD) and TS and
who received STN DBS; the patient showed a 97%
improvement in both tics and parkinsonian symptoms
after stimulation.79
VS/VC: Stimulation of VS/VC has been used as a main
target in treatment-resistant OCD; it has also been
proposed as a treatment for disorders that are
highly associated with psychiatric comorbidities,
such as TS. Based on this, a few studies have
reported that stimulation of VS/VC target moderately
improved motor severity and significantly improved
OCD.80–82 However, clinical evidences from these
targets rely on case reports and small series since
there are no controlled studies yet.
In 2005, a study showed that a TS subject who was
treated with ALIC DBS presented with only 23%
improvement on the YGTSS.82 For this reason and also
due to device problems, the authors opted to change
the target to thalamus, which resulted in more
satisfactory outcomes with a 46% decrease in the
symptoms. In 2007, Kuhn et al described another case
of TS/OCD which also improved YGTSS scores.81 Two
years later, Neuner et al reported a follow-up of 36
months after VS/VC DBS and documented close to 50%
improvement in YGSTS and significant reduction in
the YBOCS.83 Burdick et al also shared their
experience about an OCD/TS patient who was implanted
in the VS/VC target; their study revealed no
objective assessment improvement, despite the
positive opinion of the patient.84
GPe: Only case reports are available for GPe
stimulation in TS, and all of them have shown good
outcomes. In 2007, Vilela Filho et al reported GPe
DBS for TS with a double-blind assessment design.
The authors reported 81% reduction in tic scores and
84% reduction in OCD scores, 23 months after the
procedure.85 Later, Piedmonte et al also described a
case of GPe stimulation for TS and showed a 70.5%
improvement on average in anxiety and motor
symptoms.86
Go to:
Non-motor symptoms effects
Although most studies focus on the effects of motor
tic, some also have reported neuropsychological
correlates of DBS in TS.26,87–89 Besides the most
used targets (GPi and thalamus), DBS in the VS/VC,
STN, and GPe have also recorded beneficial effects
in OCD components and other psychiatric
comorbidities.
In a recent study of 15 severe TS patients with
long-term aGPi DBS, Akbarian-Tefaghi et al
investigated whether a specific anatomical site
within the aGPi correlated with optimal clinical
outcome for the measures of tics, OCB, and mood
changes. The authors observed that a region within
the ventral limbic GPi, specifically on the medial
medullary lamina in the pallidum at the level of the
AC-PC, was significantly associated with improved
tics, but changes in the mood and OCB were less
significant.46 Cury et al reported that a
23-year-old TS patient treated with CM-Pf DBS showed
very severe scores and high anxiety rate with 70.5%
improvement on YGTSS and also a significant
improvement in the anxiety scores (53%), with
clinical global impression “much improved” (from 1
to 6) after 18 months of follow-up.26
Adverse effects and complications
DBS for TS is overall considered a safe procedure;
however, some facts must be pointed out. A recent
publication from the prospective International Deep
Brain Stimulation Database and Registry presented
185 patients with refractory TS who underwent DBS
implantation from January 2012 to December 2016, at
31 institutions in 10 countries worldwide.
Thirty-five percent reported a total of 160 adverse
events during the first year of follow-up, including
dysarthria that was reported 17 times in 10 of 158
patients (6.3%), and paresthesia that was reported
15 times in 13 of 158 patients (8.2%). All of these
events were stimulation-induced and transitory
without major complications, and no deaths were
reported. The infection rate was reported to be 2.5%
(4/158), the hemorrhage rate was 1.3% (2/158), and
total explant rate at 1 year was 0.6% (1 of 158).31
Hemorrhage was described as a serious surgical
complication only in a few cases.90,91 Servello et
al in 2011 showed a higher rate of postoperative
infections of extracranial cables and generator
pockets in TS patients compared with other
movement-disorder patients (18% vs 3.7%).92
Other side effects probably stimulation-related
effects such as fatigue, apathy, lethargy, and also
maniac symptoms have been reported occasionally with
several targets.20,21,66,72,83,93 Sedative effects
have been reported mainly at high-amplitude
stimulation. There are also reports of
stimulation-induced changes in sexual behavior.72,94
Duits et al have hypothesized that the surgical
procedure or stimulation may have caused an
imbalance in the limbic and associative cortico-basal
ganglia-thalamocortical loops, thus leading to
psychiatric symptoms.93 Recently, in a long-term
follow-up of seven TS patients who underwent
bilateral DBS (CM-Pf-Voi), the authors showed that a
possible imbalance between beneficial and adverse
effects at long term can lead to either switching
the stimulator off or a proposal for an implant in a
different target.95
Conclusion
TS is a relatively rare neurodevelopmental diorder
that probably originates due to dysfunction in
motor-limbic brain circuitry linking exacerbated
anxiety to the triggering of recurring behaviors and
tics; however, the precise mechanisms are still
largely unknown. Mostly, TS starts in teenagers,
improves with conventional treatment, and tends to
disappear toward adulthood. Only severe cases, which
are uncommon, really need additional treatment.
Although DBS is not an approved therapy for TS in
most countries, positive evidence from several case
and series reports and some comparative studies
together suggest that DBS is partially effective in
alleviating symptoms in severe and
medication-resistant cases of TS. Generally,
clinical evidence has been produced by applying
chronic bilateral DBS more frequently in the CM-Pf
complex but also in the pGPi (motor GPi) or aGPi
(limbic GPi) and less frequently in VS/VC and STN
targets. This multiplicity of targets in the
literature reflects the fact that there is no
consensus on which target is the most effective.
Also, there are no defined predictors of outcome;
however, high scores in tic severity scales may be
indirectly related to better response after the DBS.
Future research involving the clinical
phenomenology, structural and functional
neuroimaging together with data from intraoperative
multi unit neuronal and multi target local field
potential recordings in TS patients will probably
allow better understanding the pathophysiology if
this complex disease, guiding interventions such as
conventional or adaptative DBS, leading to an
individualized treatment. Severe and refractory TS
is, in fact, a rare disease. In these circumstances,
it is unlikely that large controlled trials will be
performed in order to determine the efficacy of each
DBS target. It is more likely that data from
registry cohorts will provide less-qualified
evidence that will lead to a more forgiving and
humanitarian approval as it has occurred with OCD in
most countries.
Many questions are still left with no specific
answers: Is there a best DBS target for TS? Are
there specific clinical subsets of TS that would
preferentially improve with this or that target? If
so, who are the best candidates for each target? Is
adaptative DBS better than continuous stimulation?
Disclosure
Rubens G Cury has received honoraria from Medtronic,
TEVA, UCB, and Roche for lecturing and scientific
board services. Erich Talamoni Fonoff has received
honoraria for lecturing and technical assistance,
grants, personal fees, and non-financial support
from Boston Scientific. The other authors report no
conflicts of interest in this work.
References
1. Jankovic J. Tourette’s syndrome. N Engl J Med.
2001;345:1184–1192. doi: 10.1056/NEJMra010032. [PubMed]
[CrossRef] [Google Scholar]
2. Eapen V, Cavanna AE, Robertson MM. Comorbidities,
social impact, and quality of life in Tourette
syndrome. Front Psychiatry. 2016;7:97. doi:
10.3389/fpsyt.2016.00097. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
3. American Psychiatric Association, editor.
Diagnostic and Statistical Manual of Mental
Disorders: DSM-5. 5th ed. Washington, (DC): American
Psychiatric Association; 2013. [Google Scholar]
4. Leckman JF. Tourette’s syndrome. Lancet.
2002;360:1577–1586. [PubMed] [Google Scholar]
5. Bloch MH, Leckman JF. Clinical course of Tourette
syndrome. J Psychosom Res. 2009;67:497–501. doi:
10.1016/j.jpsychores.2009.09.002. [PMC free article]
[PubMed] [CrossRef] [Google Scholar]
6. Eddy CM, Rickards HE, Cavanna AE. Treatment
strategies for tics in Tourette syndrome. Ther Adv
Neurol Disord. 2011;4:25–45. doi:
10.1177/1756285610390261. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
7. Shprecher D, Kurlan R. The management of tics.
Mov Disord. 2009;24:15–24. doi: 10.1002/mds.22656.
[PMC free article] [PubMed] [CrossRef] [Google
Scholar]
8. Freeman RD, Fast DK, Burd L, Kerbeshian J,
Robertson MM, Sandor P. An international perspective
on Tourette syndrome: selected findings from 3,500
individuals in 22 countries. Dev Med Child Neurol.
2000;42:436–447. doi: 10.1017/S0012162200000839. [PubMed]
[CrossRef] [Google Scholar]
9. Malaty IA, Akbar U. Updates in medical and
surgical therapies for Tourette syndrome. Curr
Neurol Neurosci Rep. 2014;14:458. doi:
10.1007/s11910-014-0458-4. [PubMed] [CrossRef]
[Google Scholar]
10. Fasano A, Lozano AM. Deep brain stimulation for
movement disorders: 2015 and beyond. Curr Opin
Neurol. 2015;28:423–436. doi:
10.1097/WCO.0000000000000226. [PubMed] [CrossRef]
[Google Scholar]
11. Rickards H, Wood C, Cavanna AE. Hassler and
Dieckmann’s seminal paper on stereotactic
thalamotomy for Gilles de la Tourette syndrome:
translation and critical reappraisal. Mov Disord.
2008;23:1966–1972. doi: 10.1002/mds.v23:14. [PubMed]
[CrossRef] [Google Scholar]
12. Hassler R, Dieckmann G. Stereotaxic treatment of
tics and inarticulate cries or coprolalia considered
as motor obsessional phenomena in Gilles de la
Tourette’s disease. Rev Neurol (Paris)
1970;123:89–100. [PubMed] [Google Scholar]
13. Vandewalle V, van der Linden C, Groenewegen HJ,
Caemaert J. Stereotactic treatment of Gilles de la
Tourette syndrome by high frequency stimulation of
thalamus. Lancet. 1999;353:724. doi:
10.1016/S0140-6736(98)09449-5. [PubMed] [CrossRef]
[Google Scholar]
14. Jimenez-Shahed J. Design challenges for
stimulation trials of Tourette’s syndrome. Lancet
Neurol. 2015;14:563–565. doi:
10.1016/S1474-4422(15)00043-5. [PubMed] [CrossRef]
[Google Scholar]
15. Schrock LE, Mink JW, Woods DW, et al. Tourette
syndrome deep brain stimulation: a review and
updated recommendations. Mov Disord.
2015;30:448–471. doi: 10.1002/mds.26094. [PubMed] [CrossRef]
[Google Scholar]
16. Martinez JAE, Arango GJ, Fonoff ET, et al. Deep
brain stimulation of the globus pallidus internus or
ventralis intermedius nucleus of thalamus for Holmes
tremor. Neurosurg Rev. 2015;38:753–763. doi:
10.1007/s10143-015-0636-0. [PubMed] [CrossRef]
[Google Scholar]
17. Maciunas RJ, Maddux BN, Riley DE, et al.
Prospective randomized double-blind trial of
bilateral thalamic deep brain stimulation in adults
with Tourette syndrome. J Neurosurg.
2007;107:1004–1014. doi: 10.3171/JNS-07/11/1004. [PubMed]
[CrossRef] [Google Scholar]
18. Ackermans L, Duits A, van der Linden C, et al.
Double-blind clinical trial of thalamic stimulation
in patients with Tourette syndrome. Brain.
2011;134:832–844. doi: 10.1093/brain/awr044. [PubMed]
[CrossRef] [Google Scholar]
19. Kefalopoulou Z, Zrinzo L, Jahanshahi M, et al.
Bilateral globus pallidus stimulation for severe
Tourette’s syndrome: a double-blind, randomised
crossover trial. Lancet Neurol. 2015;14:595–605. doi:
10.1016/S1474-4422(15)00008-3. [PubMed] [CrossRef]
[Google Scholar]
20. Visser-Vandewalle V, Temel Y, Boon P, et al.
Chronic bilateral thalamic stimulation: a new
therapeutic approach in intractable Tourette
syndrome. Report of three cases. J Neurosurg.
2003;99:1094–1100. doi: 10.3171/jns.2003.99.6.1094.
[PubMed] [CrossRef] [Google Scholar]
21. Massano J, Sousa C, Foltynie T, Zrinzo L, Hariz
M, Vaz R. Successful pallidal deep brain stimulation
in 15-year-old with Tourette syndrome: 2-year
follow-up. J Neurol. 2013;260:2417–2419. doi:
10.1007/s00415-012-6683-3. [PubMed] [CrossRef]
[Google Scholar]
22. Sachdev PS, Mohan A, Cannon E, et al. Deep brain
stimulation of the antero-medial globus pallidus
interna for Tourette syndrome. PLoS One.
2014;9:e104926. doi: 10.1371/journal.pone.0104926.
[PMC free article] [PubMed] [CrossRef] [Google
Scholar]
23. Savica R, Stead M, Mack KJ, Lee KH, Klassen BT.
Deep brain stimulation in Tourette syndrome: a
description of 3 patients with excellent outcome.
Mayo Clin Proc. 2012;87:59–62. doi:
10.1016/j.mayocp.2011.08.005. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
24. Zhang J-G, Ge Y, Stead M, et al. Long-term
outcome of globus pallidus internus deep brain
stimulation in patients with Tourette syndrome. Mayo
Clin Proc. 2014;89:1506–1514. doi:
10.1016/j.mayocp.2014.05.019. [PubMed] [CrossRef]
[Google Scholar]
25. Schoenberg MR, Maddux BN, Riley DE, et al.
Five-months-postoperative neuropsychological outcome
from a pilot prospective randomized clinical trial
of thalamic deep brain stimulation for Tourette
syndrome. Neuromodulation. 2015;18:97–104. doi:
10.1111/ner.12233. [PubMed] [CrossRef] [Google
Scholar]
26. Cury RG, Lopez WOC, dos Santos Ghilardi MG, et
al. Parallel improvement in anxiety and tics after
DBS for medically intractable Tourette syndrome: A
long-term follow-up. Clin Neurol Neurosurg.
2016;144:33–35. doi: 10.1016/j.clineuro.2016.02.030.
[PubMed] [CrossRef] [Google Scholar]
27. Shahed J, Poysky J, Kenney C, Simpson R,
Jankovic J. GPi deep brain stimulation for Tourette
syndrome improves tics and psychiatric comorbidities.
Neurology. 2007;68:159–160. doi:
10.1212/01.wnl.0000250354.81556.90. [PubMed] [CrossRef]
[Google Scholar]
28. Nordstrom EJ, Bittner KC, McGrath MJ, Parks CR,
Burton FH. “Hyperglutamatergic
cortico-striato-thalamo-cortical circuit” breaker
drugs alleviate tics in a transgenic circuit model
of Tourette’s syndrome. Brain Res. 2015;1629:38–53.
doi: 10.1016/j.brainres.2015.09.032. [PubMed] [CrossRef]
[Google Scholar]
29. Da Cunha C, Boschen SL, Gómez AA, et al. Toward
sophisticated basal ganglia neuromodulation: review
on basal ganglia deep brain stimulation. Neurosci
Biobehav Rev. 2015;58:186–210. doi:
10.1016/j.neubiorev.2015.02.003. [PMC free article]
[PubMed] [CrossRef] [Google Scholar]
30. Müller-Vahl KR, Grosskreutz J, Prell T, Kaufmann
J, Bodammer N, Peschel T. Tics are caused by
alterations in prefrontal areas, thalamus and
putamen, while changes in the cingulate gyrus
reflect secondary compensatory mechanisms. BMC
Neurosci. 2014;15:6. doi: 10.1186/1471-2202-15-6.
[PMC free article] [PubMed] [CrossRef] [Google
Scholar]
31. Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF,
et al. Efficacy and safety of deep brain stimulation
in Tourette syndrome: the international Tourette
syndrome deep brain stimulation public database and
registry. JAMA Neurol. 2018;75:353–359. doi:
10.1001/jamaneurol.2018.2628. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
32. Florence G, Sameshima K, Fonoff ET, Hamani C.
Deep brain stimulation: more complex than the
inhibition of cells and excitation of fibers.
Neuroscientist. 2016;22:332–345. doi:
10.1177/1073858415591964. [PubMed] [CrossRef]
[Google Scholar]
33. McCairn KW, Iriki A, Isoda M. Deep brain
stimulation reduces Tic-related neural activity via
temporal locking with stimulus pulses. J Neurosci.
2013;33:6581–6593. doi:
10.1523/JNEUROSCI.3846-13.2013. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
34. Viswanathan A, Jimenez-Shahed J, Baizabal
Carvallo JF, Jankovic J. Deep brain stimulation for
Tourette syndrome: target selection. Stereotact
Funct Neurosurg. 2012;90:213–224. doi:
10.1159/000337776. [PubMed] [CrossRef] [Google
Scholar]
35. Kupsch A, Tagliati M, Vidailhet M, et al. Early
postoperative management of DBS in dystonia:
programming, response to stimulation, adverse
events, medication changes, evaluations, and
troubleshooting. Mov Disord. 2011;26(Suppl
1):S37–S53. doi: 10.1002/mds.23624. [PubMed] [CrossRef]
[Google Scholar]
36. Vernaleken I, Kuhn J, Lenartz D, et al.
Bithalamical deep brain stimulation in Tourette
syndrome is associated with reduction in
dopaminergic transmission. Biol Psychiatry.
2009;66:e15–e17. doi:
10.1016/j.biopsych.2009.06.025. [PubMed] [CrossRef]
[Google Scholar]
37. Kuhn J, Janouschek H, Raptis M, et al. In vivo
evidence of deep brain stimulation-induced
dopaminergic modulation in Tourette’s syndrome. Biol
Psychiatry. 2012;71:e11–e13. doi:
10.1016/j.biopsych.2011.09.035. [PubMed] [CrossRef]
[Google Scholar]
38. Zauber SE, Ahn S, Worth RM, Rubchinsky LL.
Oscillatory neural activity of anteromedial globus
pallidus internus in Tourette syndrome. Clin
Neurophysiol. 2014;125:1923–1924. doi:
10.1016/j.clinph.2014.01.003. [PubMed] [CrossRef]
[Google Scholar]
39. Shute JB, Okun MS, Opri E, et al.
Thalamocortical network activity enables chronic tic
detection in humans with Tourette syndrome.
Neuroimage Clin. 2016;12:165–172. doi:
10.1016/j.nicl.2016.06.015. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
40. Maling N, Hashemiyoon R, Foote KD, Okun MS,
Sanchez JC. Increased thalamic gamma band activity
correlates with symptom relief following deep brain
stimulation in humans with Tourette’s syndrome. PLoS
One. 2012;7:e44215. doi:
10.1371/journal.pone.0044215. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
41. Barow E, Neumann W-J, Brücke C, et al. Deep
brain stimulation suppresses pallidal low frequency
activity in patients with phasic dystonic movements.
Brain. 2014;137:3012–3024. doi:
10.1093/brain/awu258. [PMC free article] [PubMed] [CrossRef]
[Google Scholar]
42. Marceglia S, Rosa M, Servello D, et al. Adaptive
Deep Brain Stimulation (aDBS) for Tourette Syndrome.
Brain Sci. 2017;8:4. doi: 10.3390/brainsci8010004.
[PMC free article] [PubMed] [CrossRef] [Google
Scholar]
43. Kim JP, Min H-K, Knight EJ, et al.
Centromedian-parafascicular deep brain stimulation
induces differential functional inhibition of the
motor, associative, and limbic circuits in large
animals. Biol Psychiatry. 2013;74:917–926. doi:
10.1016/j.biopsych.2013.06.024. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
44. Singer HS, Minzer K. Neurobiology of Tourette’s
syndrome: concepts of neuroanatomic localization and
neurochemical abnormalities. Brain Dev.
2003;25(Suppl 1):S70–S84. doi:
10.1016/S0387-7604(03)90012-X. [PubMed] [CrossRef]
[Google Scholar]
45. Hartmann CJ, Lujan JL, Chaturvedi A, et al.
Tractography activation patterns in dorsolateral
prefrontal cortex suggest better clinical responses
in OCD DBS. Front Neurosci. 2015;9:519. [PMC free
article] [PubMed] [Google Scholar]
46. Akbarian-Tefaghi L, Akram H, Johansson J, et al.
Refining the deep brain stimulation target within
the limbic globus pallidus internus for Tourette
syndrome. Stereotact Funct Neurosurg.
2017;95:251–258. doi: 10.1159/000478273. [PubMed] [CrossRef]
[Google Scholar]
47. Molina R, Okun MS, Shute JB, et al. Report of a
patient undergoing chronic responsive deep brain
stimulation for Tourette syndrome: proof of concept.
J Neurosurg. 2017;29:1–7. [PMC free article] [PubMed]
[Google Scholar]
48. Chang S-Y, Kimble CJ, Kim I, et al. Development
of the Mayo investigational neuromodulation control
system: toward a closed-loop electrochemical
feedback system for deep brain stimulation. J
Neurosurg. 2013;119:1556–1565. doi:
10.3171/2013.8.JNS122142. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
49. Ackermans L, Kuhn J, Neuner I, Temel Y,
Visser-Vandewalle V. Surgery for Tourette syndrome.
World Neurosurg. 2013;80:S29.e15–S29.e22. doi:
10.1016/j.wneu.2012.06.017. [PubMed] [CrossRef]
[Google Scholar]
50. Piedad JCP, Rickards HE, Cavanna AE. What
patients with gilles de la Tourette syndrome should
be treated with deep brain stimulation and what is
the best target? Neurosurgery. 2012;71:173–192. doi:
10.1227/NEU.0b013e3182535a00. [PubMed] [CrossRef]
[Google Scholar]
51. Hamani C, Florence G, Heinsen H, et al.
Subthalamic nucleus deep brain stimulation: basic
concepts and novel perspectives. eNeuro.
2017:4:ENEURO–0140. doi:
10.1523/ENEURO.0140-17.2017. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
52. Montgomery EB. Effects of GPi stimulation on
human thalamic neuronal activity. Clin Neurophysiol.
2006;117:2691–2702. doi:
10.1016/j.clinph.2006.08.011. [PubMed] [CrossRef]
[Google Scholar]
53. Anderson JS, Dhatt HS, Ferguson MA, et al.
Functional connectivity targeting for deep brain
stimulation in essential tremor. AJNR Am J
Neuroradiol. 2011;32:1963–1968. doi:
10.3174/ajnr.A2638. [PMC free article] [PubMed] [CrossRef]
[Google Scholar]
54. Hashimoto T, Elder CM, Okun MS, Patrick SK,
Vitek JL. Stimulation of the subthalamic nucleus
changes the firing pattern of pallidal neurons. J
Neurosci. 2003;23:1916–1923. doi:
10.1523/JNEUROSCI.23-05-01916.2003. [PMC free
article] [PubMed] [CrossRef] [Google Scholar]
55. Hariz MI, Robertson MM. Gilles de la Tourette
syndrome and deep brain stimulation. Eur J Neurosci.
2010;32:1128–1134. doi:
10.1111/j.1460-9568.2010.07519.x. [PubMed] [CrossRef]
[Google Scholar]
56. Rossi PJ, Opri E, Shute JB, et al. Scheduled,
intermittent stimulation of the thalamus reduces
tics in Tourette syndrome. Parkinsonism Relat Disord.
2016;29:35–41. doi:
10.1016/j.parkreldis.2016.05.033. [PMC free article]
[PubMed] [CrossRef] [Google Scholar]
57. Ramirez-Zamora A, Giordano JJ, Gunduz A, et al.
Evolving applications, technological challenges and
future opportunities in neuromodulation: proceedings
of the fifth annual deep brain stimulation think
Tank. Front Neurosci. 2017;11:734. doi:
10.3389/fnins.2017.00734. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
58. Ackermans L, Duits A, Temel Y, et al. Long-term
outcome of thalamic deep brain stimulation in two
patients with Tourette syndrome. J Neurol Neurosurg
Psychiatr. 2010;81:1068–1072. doi:
10.1136/jnnp.2009.176859. [PubMed] [CrossRef]
[Google Scholar]
59. Bajwa RJ, de Lotbinière AJ, King RA, et al. Deep
brain stimulation in Tourette’s syndrome. Mov Disord.
2007;22:1346–1350. doi: 10.1002/mds.21234. [PubMed]
[CrossRef] [Google Scholar]
60. Servello D, Porta M, Sassi M, Brambilla A,
Robertson MM. Deep brain stimulation in 18 patients
with severe Gilles de la Tourette syndrome
refractory to treatment: the surgery and
stimulation. J Neurol Neurosurg Psychiatry.
2008;79:136–142. doi: 10.1136/jnnp.2007.124958. [PubMed]
[CrossRef] [Google Scholar]
61. Porta M, Brambilla A, Cavanna AE, et al.
Thalamic deep brain stimulation for
treatment-refractory Tourette syndrome: two-year
outcome. Neurology. 2009;73:1375–1380. doi:
10.1212/WNL.0b013e3181bd809b. [PubMed] [CrossRef]
[Google Scholar]
62. Porta M, Servello D, Zanaboni C, et al. Deep
brain stimulation for treatment of refractory
Tourette syndrome: long-term follow-up. Acta
Neurochir (Wien) 2012;154:2029–2041. doi:
10.1007/s00701-012-1497-8. [PubMed] [CrossRef]
[Google Scholar]
63. Huys D, Bartsch C, Koester P, et al. Motor
improvement and emotional stabilization in patients
with Tourette syndrome after deep brain stimulation
of the ventral anterior and ventrolateral motor part
of the thalamus. Biol Psychiatry. 2016;79:392–401.
doi: 10.1016/j.biopsych.2014.05.014. [PubMed] [CrossRef]
[Google Scholar]
64. Van der Linden C, Colle H, Vandewalle V, Alessi
G, Rijckaert D, De Waele L. Successful treatment of
tics with bilateral internal pallidum (GPi)
stimulation in a 27-year-old male patient with
Gilles de la Tourette’s syndrome (GTS) Mov Disord.
2002;17(suppl 5):S341. [Google Scholar]
65. Diederich NJ, Kalteis K, Stamenkovic M, Pieri V,
Alesch F. Efficient internal pallidal stimulation in
Gilles de la Tourette syndrome: a case report. Mov
Disord. 2005;20:1496–1499. doi: 10.1002/mds.v20:11.
[PubMed] [CrossRef] [Google Scholar]
66. Dehning S, Mehrkens J-H, Müller N, Bötzel K.
Therapy-refractory Tourette syndrome: beneficial
outcome with globus pallidus internus deep brain
stimulation. Mov Disord. 2008;23:1300–1302. doi:
10.1002/mds.21930. [PubMed] [CrossRef] [Google
Scholar]
67. Dehning S, Leitner B, Schennach R, et al.
Functional outcome and quality of life in Tourette’s
syndrome after deep brain stimulation of the
posteroventrolateral globus pallidus internus:
long-term follow-up. World J Biol Psychiatry.
2014;15:66–75. doi: 10.3109/15622975.2013.849004. [PubMed]
[CrossRef] [Google Scholar]
68. Smeets AYJM, Duits AA, Plantinga BR, et al. Deep
brain stimulation of the internal globus pallidus in
refractory Tourette syndrome. Clin Neurol Neurosurg.
2016;142:54–59. doi: 10.1016/j.clineuro.2016.01.020.
[PubMed] [CrossRef] [Google Scholar]
69. Welter ML, Houeto JL, Tezenas du Montcel S, et
al. Clinical predictive factors of subthalamic
stimulation in Parkinson’s disease. Brain.
2002;125:575–583. [PubMed] [Google Scholar]
70. Dong S, Zhuang P, Zhang X-H, Li J-Y, Li Y-J.
Unilateral deep brain stimulation of the right
globus pallidus internus in patients with Tourette’s
syndrome: two cases with outcomes after 1 year and a
brief review of the literature. J Int Med Res.
2012;40:2021–2028. doi: 10.1177/030006051204000545.
[PubMed] [CrossRef] [Google Scholar]
71. Dehning S, Feddersen B, Cerovecki A, Bötzel K,
Müller N, Mehrkens J-H. Globus pallidus internus-deep
brain stimulation in Tourette’s syndrome: can
clinical symptoms predict response? Mov Disord.
2011;26:2440–2441. doi: 10.1002/mds.23892. [PubMed]
[CrossRef] [Google Scholar]
72. Motlagh MG, Smith ME, Landeros-Weisenberger A,
et al. Lessons learned from open-label deep brain
stimulation for Tourette syndrome: eight cases over
7 Years. Tremor Other Hyperkinet Mov (N Y) 2013:3.
[PMC free article] [PubMed] [Google Scholar]
73. Nair G, Evans A, Bear RE, Velakoulis D, Bittar
RG. The anteromedial GPi as a new target for deep
brain stimulation in obsessive compulsive disorder.
J Clin Neurosci. 2014;21:815–821. doi:
10.1016/j.jocn.2013.10.003. [PubMed] [CrossRef]
[Google Scholar]
74. Cannon E, Silburn P, Coyne T, O’Maley K,
Crawford JD, Sachdev PS. Deep brain stimulation of
anteromedial globus pallidus interna for severe
Tourette’s syndrome. Am J Psychiatry.
2012;169:860–866. doi:
10.1176/appi.ajp.2012.11101583. [PubMed] [CrossRef]
[Google Scholar]
75. Martínez-Fernández R, Zrinzo L, Aviles-Olmos I,
et al. Deep brain stimulation for Gilles de la
Tourette syndrome: a case series targeting
subregions of the globus pallidus internus. Mov
Disord. 2011;26:1922–1930. doi: 10.1002/mds.23734. [PubMed]
[CrossRef] [Google Scholar]
76. Welter M-L, Houeto J-L, Thobois S, et al.
Anterior pallidal deep brain stimulation for
Tourette’s syndrome: a randomised, double-blind,
controlled trial. Lancet Neurol. 2017;16:610–619.
doi: 10.1016/S1474-4422(17)30122-9. [PubMed] [CrossRef]
[Google Scholar]
77. Welter M-L, Mallet L, Houeto J-L, et al.
Internal pallidal and thalamic stimulation in
patients with Tourette syndrome. Arch Neurol.
2008;65:952–957. doi: 10.1001/archneur.65.7.952. [PubMed]
[CrossRef] [Google Scholar]
78. Ackermans L, Temel Y, Cath D, et al. Deep brain
stimulation in Tourette’s syndrome: two targets? Mov
Disord. 2006;21:709–713. doi: 10.1002/mds.20816. [PubMed]
[CrossRef] [Google Scholar]
79. Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie
T, Limousin P. Improvement of tics after subthalamic
nucleus deep brain stimulation. Neurology.
2009;72:1787–1789. doi:
10.1212/WNL.0b013e3181a9fad1. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
80. Zabek M, Sobstyl M, Koziara H, Dzierzecki S.
Deep brain stimulation of the right nucleus
accumbens in a patient with Tourette syndrome. Case
report. Neurol Neurochir Pol. 2008;42:554–559. [PubMed]
[Google Scholar]
81. Kuhn J, Lenartz D, Mai JK, et al. Deep brain
stimulation of the nucleus accumbens and the
internal capsule in therapeutically refractory
Tourette-syndrome. J Neurol. 2007;254:963–965. doi:
10.1007/s00415-007-0648-y. [PubMed] [CrossRef]
[Google Scholar]
82. Flaherty AW, Williams ZM, Amirnovin R, et al.
Deep brain stimulation of the anterior internal
capsule for the treatment of Tourette syndrome:
technical case report. Neurosurgery. 2005;57:E403.
discussion E403. [PubMed] [Google Scholar]
83. Neuner I, Podoll K, Janouschek H, Michel TM,
Sheldrick AJ, Schneider F. From psychosurgery to
neuromodulation: deep brain stimulation for
intractable Tourette syndrome. World J Biol
Psychiatry. 2009;10:366–376. doi:
10.1080/15622970802513317. [PubMed] [CrossRef]
[Google Scholar]
84. Burdick A, Foote KD, Goodman W, et al. Lack of
benefit of accumbens/capsular deep brain stimulation
in a patient with both tics and obsessive-compulsive
disorder. Neurocase. 2010;16:321–330. doi:
10.1080/13554790903560422. [PubMed] [CrossRef]
[Google Scholar]
85. Vilela Filho O, Ragazzo P, Silva D, Ribeiro T,
Oliveira P. Bilateral globus pallidus externus deep
brain stimulation for the treatment of Tourette
syndrome: an on-going prospective controlled study.
Stereotact Funct Neurosurg. 2007;85:42–43. [Google
Scholar]
86. Piedimonte F, Andreani JCM, Piedimonte L, et al.
Behavioral and motor improvement after deep brain
stimulation of the globus pallidus externus in a
case of Tourette’s syndrome. Neuromodulation.
2013;16:55–58. doi:
10.1111/j.1525-1403.2012.00526.x. discussion 58. [PubMed]
[CrossRef] [Google Scholar]
87. Servello D, Sassi M, Brambilla A, et al. De novo
and rescue DBS leads for refractory Tourette
syndrome patients with severe comorbid OCD: a
multiple case report. J Neurol. 2009;256:1533–1539.
doi: 10.1007/s00415-009-5123-5. [PubMed] [CrossRef]
[Google Scholar]
88. Hauseux P-A, Cyprien F, Cif L, et al. Long-term
follow-up of pallidal deep brain stimulation in
teenagers with refractory Tourette syndrome and
comorbid psychiatric disorders: about three cases.
Eur J Paediatr Neurol. 2017;21:214–217. doi:
10.1016/j.ejpn.2016.06.005. [PubMed] [CrossRef]
[Google Scholar]
89. Huisman-van Dijk HM, van de Schoot R, Rijkeboer
MM, Mathews CA, Cath DC. The relationship between
tics, OC, ADHD and autism symptoms: A cross-disorder
symptom analysis in Gilles de la Tourette syndrome
patients and family-members. Psychiatry Res.
2016;237:138–146. doi:
10.1016/j.psychres.2016.01.051. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
90. Idris Z, Ghani ARI, Mar W, et al. Intracerebral
haematomas after deep brain stimulation surgery in a
patient with Tourette syndrome and low factor XIIIA
activity. J Clin Neurosci. 2010;17:1343–1344. doi:
10.1016/j.jocn.2010.01.054. [PubMed] [CrossRef]
[Google Scholar]
91. Ackermans L, Temel Y, Bauer NJC,
Visser-Vandewalle V, Dutch-Flemish Tourette Surgery
Study Group Vertical gaze palsy after thalamic
stimulation for Tourette syndrome: case report.
Neurosurgery. 2007;61:E1100. discussion E1100. [PubMed]
[Google Scholar]
92. Servello D, Sassi M, Gaeta M, Ricci C, Porta M.
Tourette syndrome (TS) bears a higher rate of
inflammatory complications at the implanted hardware
in deep brain stimulation (DBS) Acta Neurochir
(Wien) 2011;153:629–632. doi:
10.1007/s00701-010-0851-y. [PubMed] [CrossRef]
[Google Scholar]
93. Duits A, Ackermans L, Cath D, Visser-Vandewalle
V. Unfavourable outcome of deep brain stimulation in
a Tourette patient with severe comorbidity. Eur
Child Adolesc Psychiatry. 2012;21:529–531. doi:
10.1007/s00787-012-0285-6. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
94. Müller-Vahl KR, Cath DC, Cavanna AE, et al.
European clinical guidelines for Tourette syndrome
and other tic disorders. Part IV: deep brain
stimulation. Eur Child Adolesc Psychiatry.
2011;20:209–217. doi: 10.1007/s00787-011-0166-4. [PubMed]
[CrossRef] [Google Scholar]
95. Smeets AYJM, Duits AA, Leentjens AFG, et al.
Thalamic deep brain stimulation for refractory
Tourette syndrome: clinical evidence for increasing
disbalance of therapeutic effects and side effects
at long-term follow-up. Neuromodulation.
2018;21:197–202. doi: 10.1111/ner.12556. [PubMed] [CrossRef]
[Google Scholar]
96. Alho EJL, Alho ATDL, Grinberg L, et al. High
thickness histological sections as alternative to
study the three-dimensional microscopic human
sub-cortical neuroanatomy. Brain Struct Funct.
2018;223(3):1121–1132. doi:
10.1007/s00429-017-1548-2. [PMC free article] [PubMed]
[CrossRef] [Google Scholar]
97. Kaido T, Otsuki T, Kaneko Y, Takahashi A, Omori
M, Okamoto T. Deep brain stimulation for Tourette
syndrome: a prospective pilot study in Japan.
Neuromodulation. 2011;14(2):123–128. doi:
10.1111/j.1525-1403.2010.00324.x. discussion 129. [PubMed]
[CrossRef] [Google Scholar]
98. Servello D, Zekaj E, Saleh C, Lange N, Porta M.
Deep Brain Stimulation in Gilles de la Tourette
Syndrome: What Does the Future Hold? A Cohort of 48
Patients. Neurosurgery. 2016;78(1):91–100. doi:
10.1227/NEU.0000000000001004. [PubMed] [CrossRef]
[Google Scholar]
99. Houeto JL, Karachi C, Mallet L, et al.
Tourette’s syndrome and deep brain stimulation. J
Neurol Neurosurg Psychiatry. 2005;76(7):992–995.
[PMC free article] [PubMed] [Google Scholar]
100. Gallagher CL, Garell PC, Montgomery EB., Jr
Hemi tics and deep brain stimulation. Neurology.
2006;66(3):E12. [PubMed] [Google Scholar]
101. Shields DC, Cheng ML, Flaherty AW, Gale JT,
Eskandar EN. Microelectrode-guided deep brain
stimulation for Tourette syndrome: within-subject
comparison of different stimulation sites.
Stereotact Funct Neurosurg. 2008;86(2):87–91. [PubMed]
[Google Scholar]
102. Kuhn J, Lenartz D, Huff W, et al. Transient
Manic-like Episode Following Bilateral Deep Brain
Stimulation of the Nucleus Accumbens and the
Internal Capsule in a Patient With Tourette
Syndrome. Neuromodulation. 2008;11(2):128–131. [PubMed]
[Google Scholar]
103. Kuhn J, Gaebel W, Klosterkoetter J, Woopen C.
Deep brain stimulation as a new therapeutic approach
in therapy-resistant mental disorders: ethical
aspects of investigational treatment. Eur Arch
Psychiatry Clin Neurosci. 2009;259(Suppl
2):S135–S141. doi: 10.1007/s00406-009-0055-8.
Review. [PubMed] [CrossRef] [Google Scholar]
104. Dueck A, Wolters A, Wunsch K, et al. Deep brain
stimulation of globus pallidus internus in a
16-year-old boy with severe tourette syndrome and
mental retardation. Neuropediatrics.
2009;40(5):239–242. doi: 10.1055/s-0030-1247519. [PubMed]
[CrossRef] [Google Scholar]
105. Foltynie T, Martinez-Torres I, Zrinzo L, et al.
Improvement in vocal & motor tics following dbs
motor Gpi for Tourette sindrome, not accompanied by
subjective improvement in quality of life – Case
Report. Mov Disord. 2009;24:S497–S498. [Google
Scholar]
106. Marceglia S, Servello D, Foffani G, et al.
Thalamic single-unit and local field potential
activity in Tourette syndrome. Mov Disord.
2010;25(3):300–308. [PubMed] [Google Scholar]
107. Lee MWY, Au-Yeung MM, Hung KN, Wong CK. Deep
brain stimulation in a Chinese Tourette’s syndrome
patient. Hong Kong Med J. 2011;17(2):147–150. [PubMed]
[Google Scholar]
108. Kuhn J, Bartsch C, Lenartz D, Huys D, Daumann
J, Woopen C, et al. Clinical effectiveness of
unilateral deep brain stimulation in Tourette
syndrome. Transl Psychiatry. 2011;1:e52. [PMC free
article] [PubMed] [Google Scholar]
109. Hwynn N, Tagliati M, Alterman RL, et al.
Improvement of both dystonia and tics with 60 Hz
pallidal deep brain stimulation. Int J Neurosci.
2012;122(9):519–522. [PubMed] [Google Scholar]
110. Huasen B, McCreary R, Evans J, Potter G,
Silverdale M. Cervical myelopathy secondary to
Tourette’s syndrome managed by urgent deep brain
stimulation. Mov Disord. 2014;29(4):452–453. [PubMed]
[Google Scholar]
111. Patel N, Jimenez-Shahed J. Simultaneous
improvement of tics and parkinsonism after pallidal
DBS. Parkinsonism Relat Disord.
2014;20(9):1022–1023. [PubMed] [Google Scholar]
112. Zekaj E, Saleh C, Porta M, Servello D.
Temporary deep brain stimulation in Gilles de la
Tourette syndrome: A feasible approach? Surg Neurol
Int. 2015;6:122. [PMC free article] [PubMed] [Google
Scholar]
113. Testini P, Zhao CZ, Stead M, Duffy PS, Klassen
BT, Lee KH. Centromedian-Parafascicular Complex Deep
Brain Stimulation for Tourette Syndrome: A
Retrospective Study. Mayo Clin Proc.
2016;91(2):218–225. [PMC free article] [PubMed]
[Google Scholar]
114. Dwarakanath S, Hegde A, Ketan J, et al. “I
swear, I can’t stop it!” – A case of severe
Tourette’s syndrome treated with deep brain
stimulation of anteromedial globus pallidus interna.
Neurol India. 2017;65(1):99–102. [PubMed] [Google
Scholar]
115. Smeets AYJM, Duits AA, Plantinga BR, et al.
Deep Brain Stimulation of the internal globus
pallidus in refractory Tourette Syndrome. Clin
Neurol Neurosurg. 2016;142:54–59. [PubMed] [Google
Scholar]
116. Okun MS, Foote KD, Wu SS, et al. A trial of
scheduled deep brain stimulation for Tourette
syndrome: moving away from continuous deep brain
stimulation paradigms. JAMA Neurol.
2013;70(1):85–94. [PubMed] [Google Scholar]
117. Vandewalle V, van der Linden C, Groenewegen HJ,
Caemaert J. Stereotactic treatment of Gilles de la
Tourette syndrome by high frequency stimulation of
thalamus. Lancet. 1999;353:724. [PubMed] [Google
Scholar]
 SUMMARY AND RECOMMENDATIONS — Tourette syndrome
(TS) is a common movement and neurobehavioral
disorder in children characterized by multiple motor
and vocal tics. The genetic basis of TS remains
elusive, but several loci have been identified as
candidate susceptibility regions. A mutation in the
Slit and Trk-like 1 (SLITRK1) gene on chromosome
13q31.1 is of particular interest. The onset of TS
is typically between age 2 and 15 years and occurs
by 11 years of age in 96 percent of patients.
However, the diagnosis may be delayed until 21 years
in some cases. Common comorbid conditions in TS
include attention deficit hyperactivity disorder
(ADHD), obsessive compulsive disorder (OCD),
disordered impulse control and other behavioral
problems. The diagnosis of TS is based on the
clinical features, particularly the presence of
multiple motor and vocal tics, with onset before age
21. The diagnosis is often supported by the presence
of coexisting behavioral disorders such as ADHD
and/or OCD, and a family history of similar
symptoms. Pharmacotherapy is indicated only when
symptoms of TS are interfering with social
interactions, school or job performance, or
activities of daily living. For patients with TS and
bothersome tics, we recommend drugs such as
fluphenazine starting at 1 mg daily, pimozide
starting at 2 mg daily, or tetrabenazine starting at
12.5 mg daily. For patients with TS who have only
focal motor or vocal tics, we recommend treatment
with botulinum toxin injections into the affected
muscles. For patients who have TS and ADHD, we
recommend stimulants such as methylphenidate
starting at 5 mg daily or dextroamphetamine starting
at 5 mg daily. For patients who have TS and
predominant behavioral symptoms, particularly
impulse control problems and rage attacks, we
recommend clonidine starting at 0.1 mg daily or
guanfacine starting at 1 mg daily. For patients who
have TS and OCD, we recommend serotonergic drugs
such as fluoxetine starting at 20 mg daily.
SUMMARY AND RECOMMENDATIONS — Tourette syndrome
(TS) is a common movement and neurobehavioral
disorder in children characterized by multiple motor
and vocal tics. The genetic basis of TS remains
elusive, but several loci have been identified as
candidate susceptibility regions. A mutation in the
Slit and Trk-like 1 (SLITRK1) gene on chromosome
13q31.1 is of particular interest. The onset of TS
is typically between age 2 and 15 years and occurs
by 11 years of age in 96 percent of patients.
However, the diagnosis may be delayed until 21 years
in some cases. Common comorbid conditions in TS
include attention deficit hyperactivity disorder
(ADHD), obsessive compulsive disorder (OCD),
disordered impulse control and other behavioral
problems. The diagnosis of TS is based on the
clinical features, particularly the presence of
multiple motor and vocal tics, with onset before age
21. The diagnosis is often supported by the presence
of coexisting behavioral disorders such as ADHD
and/or OCD, and a family history of similar
symptoms. Pharmacotherapy is indicated only when
symptoms of TS are interfering with social
interactions, school or job performance, or
activities of daily living. For patients with TS and
bothersome tics, we recommend drugs such as
fluphenazine starting at 1 mg daily, pimozide
starting at 2 mg daily, or tetrabenazine starting at
12.5 mg daily. For patients with TS who have only
focal motor or vocal tics, we recommend treatment
with botulinum toxin injections into the affected
muscles. For patients who have TS and ADHD, we
recommend stimulants such as methylphenidate
starting at 5 mg daily or dextroamphetamine starting
at 5 mg daily. For patients who have TS and
predominant behavioral symptoms, particularly
impulse control problems and rage attacks, we
recommend clonidine starting at 0.1 mg daily or
guanfacine starting at 1 mg daily. For patients who
have TS and OCD, we recommend serotonergic drugs
such as fluoxetine starting at 20 mg daily.
|